An exploration group led by Prof. Huang Qing from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences has utilized infrared and Raman spectroscopy to distinguish lysine acetylation highlights, giving a hypothetical and exploratory reason for the examination of protein acetylation structures in organic frameworks.
Their outcomes were published in Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.
Acetylation is a typical and significant protein change in science and plays a vital administrative role in cell digestion. The first is N-acetylation, which is well defined for lysine buildups, and the second is N-terminal acetylation, which can occur on various amino corrosive deposits.
As of now, N-terminal acetyltransferase is by and large used to mark lysine buildups as acetylated, yet the precision of this strategy is as yet dubious. Recognizing protein acetylation at a sub-atomic level is one of the ebb and flow research difficulties, and the key is to precisely limit and describe the acetylation of lysine to acquire a reasonable and orderly comprehension.
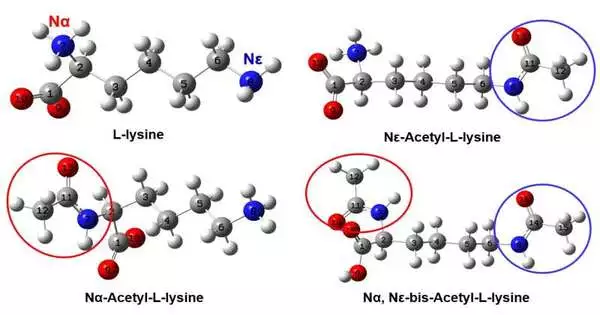
To take care of this issue, the examination group deliberately concentrated on the underlying changes and compared vibrational phantom attributes of the three acetylation types (N-Ace-Lys, N-Ace-Lys and NN-Ace-Lys) of L-lysine through infrared and Raman spectroscopy analyses and thickness capability hypothesis estimations. They viewed that as the infrared and Raman trademark groups of the amide bunch, carboxyl groups, and different groups can be utilized to successfully distinguish different acetylation types.
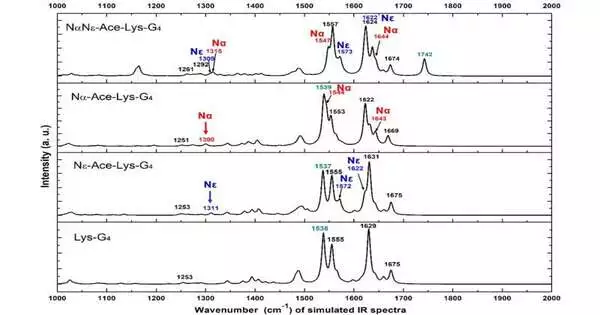
Base: a hypothetically determined infrared spectra of the Lys-G4 polypeptide and its three acetylated particles. Red is N-acetyl, blue is N-acetyl.
At the end of the day, whether lysine was acetylated can be told from the qualities of infrared and Raman spectra, as well as the sort of lysine.
Simultaneously, the group’s vibrational spectroscopy distinguishing proof system for acetylation was additionally confirmed in the peptide model.
This examination gives the vibrational mode investigation of acetylated lysine and proposes an otherworldly distinguishing proof and another portrayal technique for lysine acetylation.
More information: Guohua Yao et al, Theoretical and experimental study of the infrared and Raman spectra of L-lysine acetylation, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy (2022). DOI: 10.1016/j.saa.2022.121371