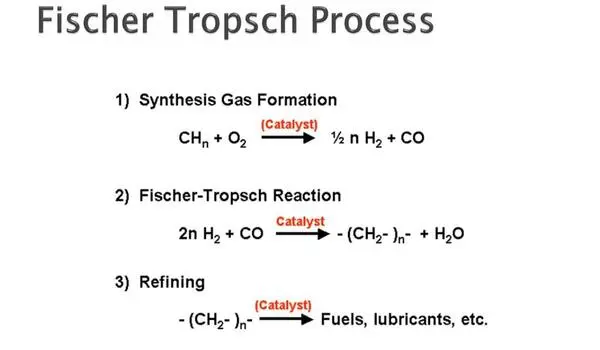
A basic finding regarding the Fischer Tropsch process, a catalytic reaction used in industry to convert coal, natural gas, or biomass to liquid fuels, may one day lead to more efficient fuel production.
Researchers at Washington State University uncovered previously undiscovered self-sustained oscillations in the Fischer Tropsch process. They discovered that, unlike many catalytic reactions, which have a single steady state, this reaction alternates between high and low activity levels. The research, published in Science, suggests that these well-controlled oscillatory states could be exploited in the future to improve reaction rates and yields of desired products.
“Usually, rate oscillations with large variations in temperature are unwanted in the chemical industry because of safety concerns,” said corresponding author Norbert Kruse, Voiland Distinguished Professor in WSU’s Gene and Linda Voiland School of Chemical Engineering and Bioengineering. “In the present case, oscillations are under control and mechanistically well understood. With such a basis of understanding, both experimentally and theoretically, the approach in research and development can be completely different — you really have a knowledge-based approach, and this will help us enormously.”
In the present case, oscillations are under control and mechanistically well understood. With such a basis of understanding, both experimentally and theoretically, the approach in research and development can be completely different – you really have a knowledge-based approach, and this will help us enormously.
Norbert Kruse
Although the Fischer-Tropsch process is widely employed in fuel and chemical manufacturing, researchers have little grasp of how the complex catalytic conversion process operates. The process employs a catalyst to convert two simple molecules, hydrogen and carbon monoxide, into long chains of molecules — hydrocarbons, which are frequently used in everyday life.
While a trial-and-error approach has been used in fuel and chemical research and development for over a century, researchers will now be able to design catalysts more intentionally and tune the reaction to induce oscillatory states that may improve catalytic performance.
The researchers first came upon the oscillations by accident after graduate student Rui Zhang approached Kruse with a problem: he wasn’t able to stabilize the temperature in his reaction. As they studied it together, they discovered the surprising oscillations.
“That was pretty funny,” Kruse said. “He showed it to me, and I said, ‘Rui, congratulations, you have oscillations! And then we developed this story more and more.”
The researchers revealed not just that the reaction produces rhythmic reaction states, but also why this occurs. That is, as the temperature of the reaction rises due to heat production, the reactant gases lose contact with the catalyst surface, slowing their reaction and lowering the temperature. When the temperature drops sufficiently, the concentration of reactant gases on the catalyst surface increases, and the reaction resumes its normal pace. As a result, the temperature rises to close the cycle.
For the study, the researchers demonstrated the reaction in a lab employing a frequently used cobalt catalyst, conditioned by adding cerium oxide, and then modeled how it worked. Co-author Pierre Gaspard at the Université Libre de Bruxelles developed a reaction scheme and theoretically imposed periodically changing temperatures to replicate the experimental rates and selectivities of the reaction.
“It’s so beautiful that we were able to model that theoretically,” said corresponding author Yong Wang, Regents Professor in WSU’s Voiland School and Zhang’s co-advisor. “The theoretical and the experimental data nearly coincided.”
Kruse has been studying oscillatory reactions for almost thirty years. The discovery of oscillatory behavior with the Fischer-Tropsch reaction was particularly surprising because the reaction is extremely complicated mechanically.
“We have a lot of frustration in our research because things aren’t going the way you think they should, but then there are moments that you can’t describe,” Kruse explained. “It’s so rewarding, but ‘rewarding’ is a weak expression for the excitement of having had this fantastic breakthrough.”





