Film innovations can provide productive filtration of hazy water sources to address a developing water shortage. Notwithstanding, when designers foster cutting-edge films, they are obliged by an absence of selectivity compared with high porousness. In addition, the current membranes can only remove neutral solutes with low molecular weights effectively and are susceptible to degradation from oxidants that are part of water treatment.
In another report currently distributed in Science Advances, Duong Nguyen and an exploration group in ecological and design design, synthetic and natural design, at the College of Colorado and the College of English Columbia in the U.S. and Canada fostered a water desalination strategy by utilizing applied strain to drive fume transport through layers.
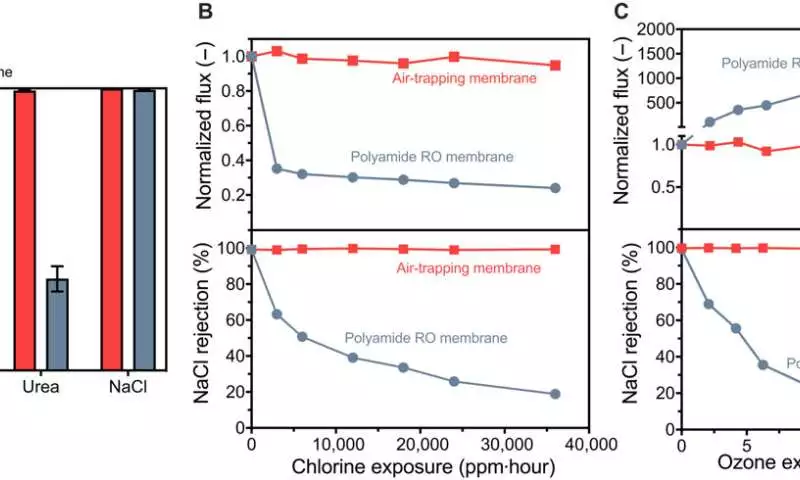
The team created membranes with water permeabilities that were greater than those of commercial membranes and that were sensitive to chlorine and ozone oxidants without compromising salt elimination. These membranes had layers that were less than 200 nm thick. The results prompted a progressed portrayal of the vanishing conduct for high-throughput, super-specific filtration.
Desalination of water
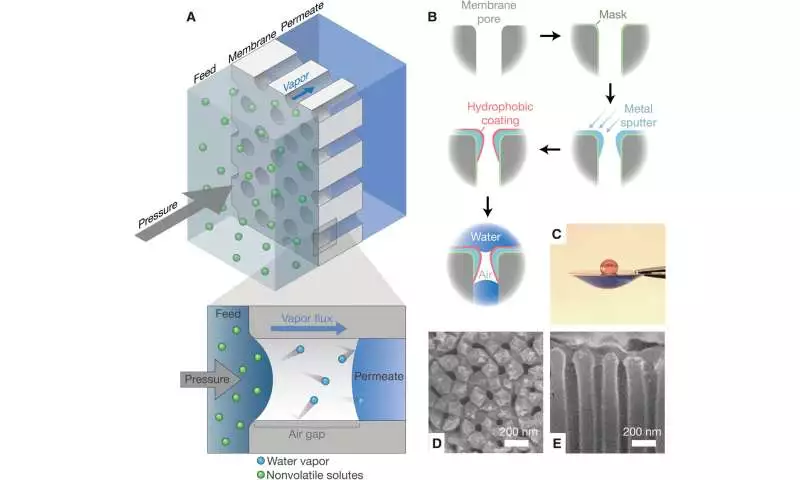
Due to water scarcity caused by climate change and rising demand, non-traditional water sources must be utilized. To securely utilize the source, analysts should wipe out virtually undeniably disintegrated constituents from tainted water. For water reuse and desalination, a number of approaches, such as reverse osmosis, have emerged. In this work, Nguyen and partners introduced a proof-of-idea technique to involve pressure refining by catching air in a hydrophobic film.
The applied strain drove dissipation inside the framework for gas-stage dissemination through pores. The materials researchers created nanoporous layers with sub-200 nm thickness to further develop test transport and desalination execution. Even when the membranes were subjected to chlorine and ozone disinfectants, this construction enabled the performance of desalination to be maintained.
Designing nanoscale air holes for pressure-driven refining
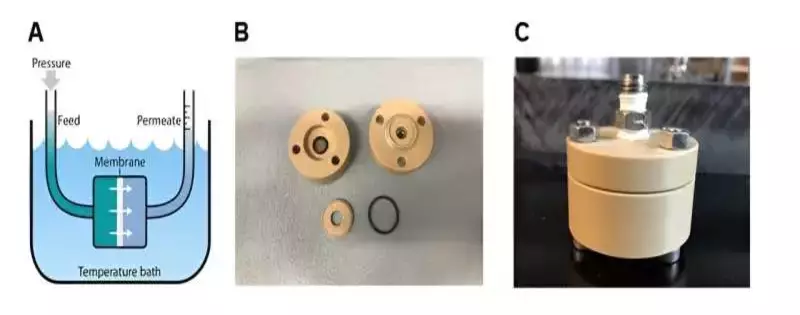
The specialists utilized air-catching layers as a proof-of-idea to test permeable anodic aluminum oxide substrates coated with a hydrophobic coat. The group restricted the coat to a sub-micron layer on the top surface of the film by utilizing veiling, metal spluttering, and hydrophobic covering with fluorinated alkyl silane. Specialists and structural architects had not recently utilized air-catching films in compressed applications since fluid water can enter the pores without thinking twice about the salt selectivity of the layer.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
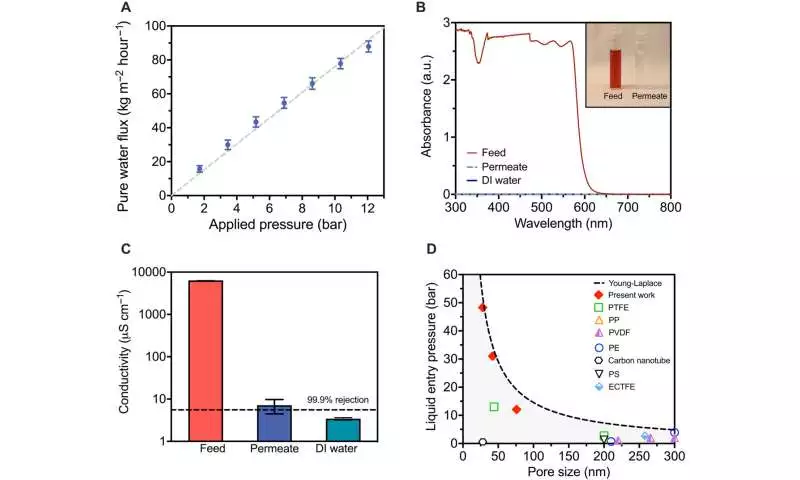
At high hydraulic pressures, the membrane’s sub-100 nm pore size resisted wetting. Long-haul tests directed for seven days demonstrated the way that the salt disposal from layers could be reliably kept up with. The group diminished the film pore sizes to work at water-driven pressures without wetting the pores. These developments held pore sizes proper for seawater treatment.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Achieving water porousness and salt end
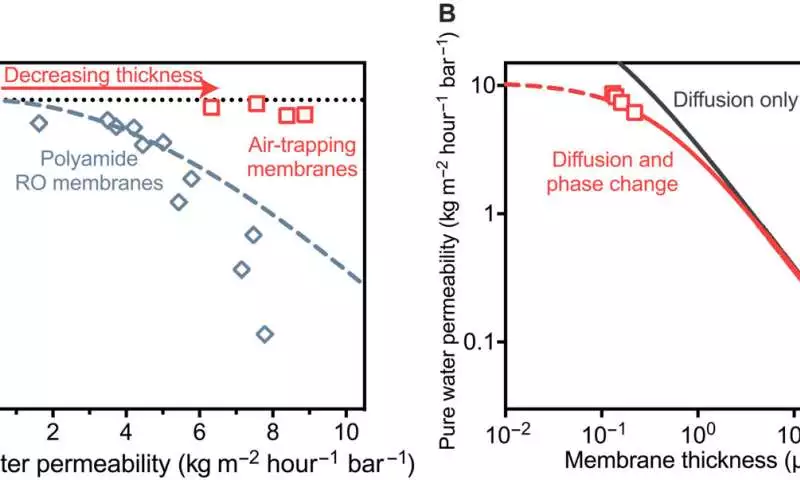
The current cutting-edge films present a tradeoff between water porousness and water-salt selectivity, by which an addition in water penetrability prompted a misfortune in salt dismissal. In this work, the group noticed how a gas-fluid stage change can increment water penetrability without forfeiting the water-salt selectivity by diminishing the thickness of the air layer.
Membranes have significantly higher water permeabilities than previously thought because of this property. The researchers surpassed the assumptions for this study when they showed exploratory proof for the conceivable outcomes of air-catching layers to arrive at water permeabilities appropriate for effective water desalination.
Chemical resiliency and solute elimination The team used a vapor layer with different selective properties as a separation barrier rather than a thin polymer film. They tested the membrane’s ability to remove NDMA, urea, and boron, three contaminants. The membranes eliminated boron at 99.1 percent and urea at 98.1 percent, respectively, demonstrating that both solutes were removed nearly completely. The results were different from commercial membranes, which had significantly lower filtration levels.
The air layer, which served as a near-impermeable filter membrane for non-volatile solutes, was responsible for the improved outcomes. Nguyen and colleagues were able to use hydrophobic membrane materials that were resistant to oxidation thanks to the air layer. After exposing the engineered membranes to scanning electron microscopy and contact angle analysis, the researchers were able to interestingly confirm that their structure and chemistry remained unchanged.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Viewpoint
Along these lines, Duong Nguyen and partners utilized a tension-driven refining process for water decontamination, with applied pressure used to drive fumes through an air-catching layer. By utilizing confirmation-of-idea tries, the researchers achieved higher filtration levels of nonvolatile solutes, including sodium chloride, boron, and urea. The material’s exhibition stayed healthy upon openness to supported degrees of high convergences of chlorine and ozone.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
This work frames the basic standards of tension-driven refining strategies, despite the fact that holes actually exist in the information on the hidden vanishing process. These layers give a stage to concentrate extensively on the vanishing peculiarities at the air-film interface.
More information: Duong T. Nguyen et al, Pressure-driven distillation using air-trapping membranes for fast and selective water purification, Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Menachem Elimelech et al, The Future of Seawater Desalination: Energy, Technology, and the Environment, Science (2011). DOI: 10.1126/science.1200488