The electrolysis of water to produce oxygen for breathing on bases on the moon or Mars could be up to 11% less efficient due to their lower gravity compared to Earth, according to UK researchers. The finding supports the approach’s viability, but it emphasizes the importance of limiting the effects of low gravity in the development of such systems for future missions.
Scientists from The University of Manchester and The University of Glasgow have provided new insight into the possibility of establishing a pathway to generate oxygen for humans to potentially call the Moon or Mars “home” for extended periods of time.
In an era when space travel is more feasible than ever before, developing a reliable source of oxygen could aid humanity in establishing habitable habitats beyond Earth. Electrolysis is a well-known potential method that involves passing electricity through a chemical system to drive a reaction. It can be used to extract oxygen from lunar rocks or to split water into hydrogen and oxygen. This has the potential to be useful for both life support systems and in-situ rocket propellant production.
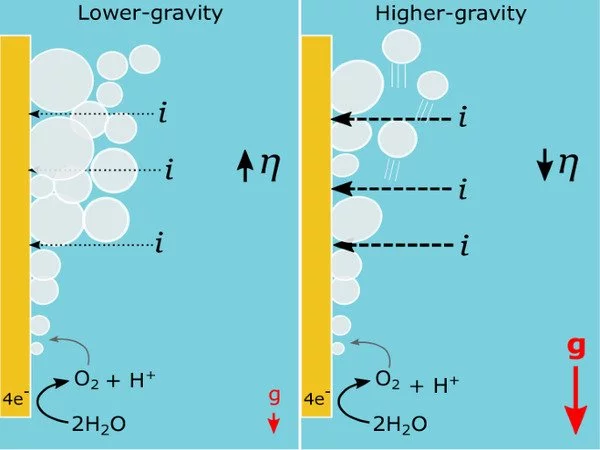
Until now, however, it had not been thoroughly investigated how lower gravitational fields on the Moon (1/6th of Earth’s gravity) and Mars (1/3rd of Earth’s gravity) might affect gas-evolving electrolysis when compared to known conditions on Earth. Lower gravity can have a significant impact on electrolysis efficiency, as bubbles can remain stuck to electrode surfaces and create a resistive layer.
We designed and built a small centrifuge that could generate a range of gravity levels relevant to the Moon and Mars, and operated it during microgravity on a parabolic flight, to remove the influence of Earth’s gravity.
Gunter Just
The moon and Mars have gravity of 0.16g and 0.37g, respectively, compared to 1g on Earth. Scientists knew that zero-g electrolysis of water is less efficient because oxygen bubbles are less buoyant and collect around electrodes. However, due to technical difficulties, no one had studied the process at micro-g levels ranging from 0.01g to 1g, and thus how lunar or Martian gravity would affect oxygen production.
Now, a team led by Mark Symes at the University of Glasgow, UK, have conducted water electrolysis experiments within this range for the first time. New research published in Nature Communications demonstrates how a team of researchers from The University of Manchester and the University of Glasgow undertook experiments to determine how the potentially life-giving electrolysis method acted in reduced gravity conditions.
“We designed and built a small centrifuge that could generate a range of gravity levels relevant to the Moon and Mars, and operated it during microgravity on a parabolic flight, to remove the influence of Earth’s gravity,” said Gunter Just, the project’s lead engineer.
When performing an experiment in the lab, it is impossible to escape the gravity of Earth; however, in the almost zero-g environment of the aircraft, our electrolysis cells were only influenced by centrifugal force, allowing us to tune the gravity-level of each experiment by changing the rotation speed. The centrifuge had four 25 cm arms that each held an electrolysis cell equipped with a variety of sensors, so during each parabola of around 18 seconds we did four simultaneous experiments on the spinning system.
“We also operated the same experiments on the centrifuge between 1 and 8 g in the laboratory. In this configuration we had the arms swinging so that the downwards gravity was accounted for.It was found that the trend observed below 1 g was consistent with the trend above 1 g, which experimentally verified that high gravity platforms can be used to predict electrolysis behaviour in lunar gravity, removing the limitations of needing costly and complex microgravity conditions. In our system, we found that 11% less oxygen was produced in lunar gravity, if the same operating parameters were used as on Earth.”
The additional power requirement was more modest at around 1 %. These specific values are only relevant to the small test cell but demonstrate that the reduced efficiency in low gravity environments must be taken into account when planning power budgets or product output for a system operating on the Moon or Mars. If the impact on power or product output was deemed too large for a system to function properly, some adaptations could be made that may reduce the effect of gravity, such as using a specially structured electrode surface or introducing flow or stirring.





