The blending of natural mixtures and polymers is at the center of many assembling ventures. The new “zapping blend” strategies that can consolidate traditional engineered science with electrochemistry are a bit nearer to a supportable tomorrow. These responses don’t need potentially unsafe compound reagents. They accomplish natural amalgamation by basically utilizing electrons from an electric power source to direct redox responses.
Aside from being harmless to the ecosystem, these responses can likewise be made pretty specific by tweaking the electric possibilities. In any case, their reliance on a power supply restricts their application in unpowered areas like aviation and the remote ocean.
The answer to this self-disconnected issue was introduced by a group of scientists led by Prof. Shinsuke Inagi from the Tokyo Institute of Technology (Tokyo Tech), Japan. In their new review distributed in Communications Chemistry, the group gave a proof-of-idea for electrochemical polymerization of natural fragrant monomers without an outside power supply. Prof. Inagi makes sense of, “We have seen an immense jump in the improvement of electrochemical reactors for completing natural union, yet the greater part of them require a power source.” We needed to construct a power-free framework to make the interaction more open. Furthermore, we tracked down the solution to our mission in streaming potential-driven electrochemistry. “
“The entire globe is working to make important industrial processes more environmentally friendly and cleaner. Because organic synthesis lies at the heart of many chemical companies, we attempted to build an electrosynthesis process that uses little resources and contributes to long-term development goals.”
Prof. Inagi.
What precisely is this streaming possibility that Prof. Inagi makes reference to?
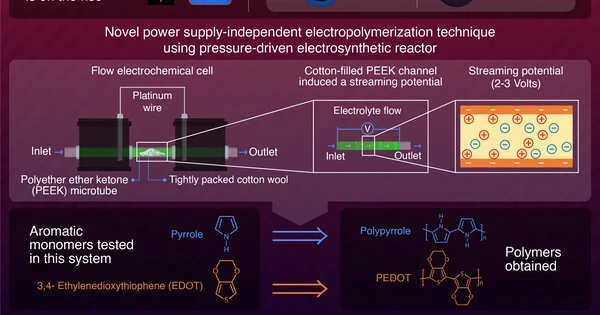
At the point when an electrolyte courses through a microchannel, a tension contrast is made because of this development. This causes an uneven charge, which leads to a streaming potential. The group utilized a custom two-chambered polyether ether ketone (or PEEK) cell associated with platinum wires and a PEEK microtube for their investigations. This PEEK microtube was firmly loaded up with cotton fleece to make a tension drop. When they put an electrolyte through the microtube, it produced a streaming potential that could give sufficient energy to drive the ideal compound responses.
At the point when the phone was working, the terminals in the two-chambered cell experienced both upstream and downstream streaming potential, which empowered the phone to act like something many refer to as a split bipolar cathode (BPE). This BPE arrangement, joined by the produced streaming capability of 2-3 volts, was responsible for making conditions helpful for the redox responses of natural monomers.
To test the polymerization capacities of this arrangement, the group picked two fragrant natural mixtures: Pyrrole (Py) and 3,4-Ethylenedioxythiophene (EDOT). Both these monomers were effectively electropolymerized into polypyrrole (PPy) and poly-EDOT (PEDOT) individually, without utilizing any outside power source.
This new strain-driven, harmless to the ecosystem, power supply-autonomous reactor opens up new roads for zapping union responses. The findings of this study can also be useful in designing new electrochemical reactors for the combination of valuable natural mixtures and polymers. “The whole world is attempting to make fundamental modern cycles greener and cleaner. “Since natural amalgamation is at the core of numerous synthetic ventures, we attempted to create an electrosynthesis interaction that requires the least assets and contributes towards the practical improvement objectives,” finishes up Prof. Inagi.