Scientists demonstrated in a mouse study that gene-edited cellular therapeutics can successfully treat cardiovascular and pulmonary diseases, potentially paving the way for the development of less expensive cellular therapies to treat diseases for which there are currently few viable options.
UC San Francisco researchers have demonstrated that gene-edited cellular therapeutics can successfully treat cardiovascular and pulmonary diseases, potentially paving the way for the development of less expensive cellular therapies to treat diseases for which there are currently few viable options.
The study, conducted in mice, is the first in the emerging field of regenerative cell therapy to demonstrate that products derived from specially engineered induced pluripotent stem cells known as “HIP” cells can be used to treat major diseases while evading the immune system. The findings disrupt the immune response, which is a major cause of transplant failure and a barrier to using engineered cells for therapy.
We demonstrated that immune-engineered HIP cells consistently avoid immune rejection in mice with different tissue types, a situation similar to unrelated human transplantation. Without the use of immunosuppressive drugs, this immune evasion was maintained in diseased tissue and tissue with poor blood supply.
Tobias Deuse
“We demonstrated that immune-engineered HIP cells consistently avoid immune rejection in mice with different tissue types, a situation similar to unrelated human transplantation. Without the use of immunosuppressive drugs, this immune evasion was maintained in diseased tissue and tissue with poor blood supply “Tobias Deuse, MD, the Julien I.E. Hoffman, M.D. Endowed Chair in Cardiac Surgery and one of the study’s first authors, said.
Deuse’s research is an example of “living therapeutics,” a new branch of medicine in which treatments are defined broadly as living human and microbial cells that are selected, modified, or engineered to treat or cure disease. The findings were published in the Proceedings of the National Academy of Sciences.
“Universal Stem Cells” Avoid Immune Detection
The scientists report that the prospects of creating specialized cells in a dish that can be transplanted into patients to treat various diseases are promising. The immune system, on the other hand, would immediately recognize and reject cells recovered from another individual. As a result, some scientists believe that custom cell therapeutics must be created from the ground up, beginning with a blood sample from each individual patient.
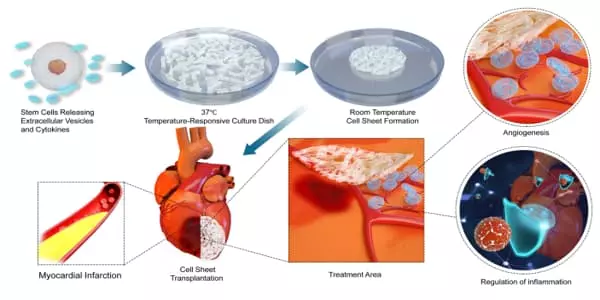
The UCSF research team took a different approach, using gene editing to create ‘universal stem cells’ (dubbed HIP cells) that are immune-resistant and can be used to create “universal cell therapeutics.”
The researchers investigated the ability of these cells to treat three major diseases affecting different organ systems: peripheral artery disease, chronic obstructive pulmonary disease caused by alpha1-antitrypsin deficiency, and heart failure, which is becoming a global epidemic with over 5.7 million patients in the United States alone and approximately 870,000 new cases each year.
The researchers implanted specialized, immune-engineered HIP cells into mice with each of these conditions and were able to demonstrate that the cell therapeutics could alleviate peripheral artery disease in the hindlimbs, prevent the development of lung disease in mice with alpha1-antitrypsin deficiency, and alleviate heart failure in mice following myocardial infarction.
To improve the translational aspect of this proof-of-concept study, the researchers assessed the efficacy of the treatment using standard parameters for human clinical trials focusing on outcome and organ function.
The Promise of an Affordable Option
Deuse, who is also the surgical director of the Transcatheter Valve Program and the director of Minimally Invasive Cardiac Surgery, intends to investigate the potential of these universal stem cells in the treatment of other endocrine and cardiovascular conditions. Because of the novelty of the approach, he noted that a careful and measured introduction into clinical trials will be critical. He believes that once more information about human safety is available, it will be easier to predict when treatments based on HIP cells will be approved and available to patients.
According to Deuse, one of the major advantages of this approach is the low cost of the immune engineering strategy. It would reduce the cost of producing universal, high-quality cell therapeutics, allowing for future treatment of larger patient populations and facilitating access for patients from underserved communities.
“A therapeutic must be affordable in order to have a broad impact,” said Deuse. “That is why we place such a premium on immune engineering and the creation of universal cells. When costs are reduced, access for all patients in need improves.”





