There are presently not many great therapy choices for glioblastoma, a forceful kind of mind disease with a high casualty rate. One explanation why the infection is so hard to treat is that most chemotherapy drugs can’t enter the veins that encompass the mind.
A group of MIT specialists is currently creating drug-conveying nanoparticles that seem to get into the mind more efficiently than drugs given all alone. Utilizing a human tissue model they planned, which precisely reproduces the blood-mind obstruction, the scientists demonstrated the way that the particles could get into cancer cells and kill glioblastoma cells.
Numerous potential glioblastoma medicines have shown progress in creature models, but at that point, they wound up flopping in clinical preliminaries. This recommends that a superior sort of demonstrating is required, says Joelle Straehla, the Charles W. and Jennifer C. Johnson Clinical Investigator at MIT’s Koch Institute for Integrative Cancer Research, an educator at Harvard Medical School, and a pediatric oncologist at Dana-Farber Cancer Institute.
“We’re hopeful that by testing these nanoparticles in a much more realistic model, we’ll be able to save a lot of time and energy that’s been lost in the clinic attempting things that don’t work. Unfortunately, hundreds of trials for this form of brain tumor have had poor outcomes.”
Joelle Straehla Clinical Investigator at MIT
“We are trusting that by testing these nanoparticles in a considerably more reasonable model, we can remove a great deal of the significant investment that is squandered on difficult things in the center that don’t work,” she says. “Sadly, for this kind of mind cancer, there have been many preliminary that have had adverse outcomes.”
Straehla and Cynthia Hajal, a postdoc at Dana-Farber, are the lead creators of the review, which appears this week in the Proceedings of the National Academy of Sciences. Paula Hammond, an MIT Institute Professor and head of the Department of Chemical Engineering; an individual from the Koch Institute; and Roger Kamm, the Cecil and Ida Green Distinguished Professor of Biological and Mechanical Engineering, are the senior creators of the paper.
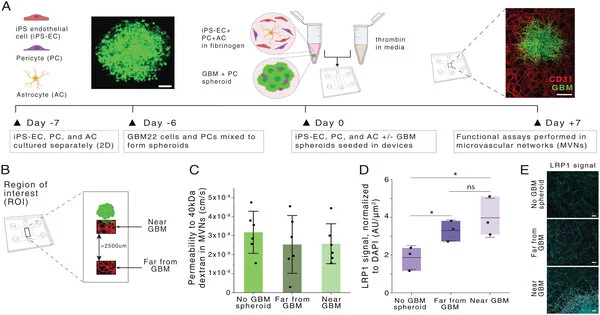
Demonstrating the blood-mind obstruction
Quite a while back, Kamm’s lab started dealing with a microfluidic model of the mind and the veins that make up the blood-cerebrum boundary.
Since the mind is a particularly fundamental organ, the veins encompassing the cerebrum are significantly more prohibitive than other veins in the body to keep out possibly hurtful particles.
To copy that construction in a tissue model, the specialists developed patient-determined glioblastoma cells in a microfluidic gadget. Then, at that point, they utilized human endothelial cells to develop veins in little cylinders encompassing the circle of cancer cells. The model additionally incorporates pericytes and astrocytes, two cell types that are associated with shipping particles across the blood-brain obstruction.
While Hajal was chipping away at this model as an alumni understudy in Kamm’s lab, she got associated with Straehla, then a postdoc in Hammond’s lab, who was keen on tracking down better approaches to demonstrate nanoparticle drug conveyance to the cerebrum. Getting drugs across the blood-brain obstruction is essential for further developing therapy for glioblastoma, which is generally treated with a mix of a medical procedure, radiation, and the oral chemotherapy drug temozolomide. The five-year endurance rate for the infection is under 10%.
Hammond’s lab spearheaded a method called layer-by-layer gathering, which they can use to make surface-functionalized nanoparticles that convey drugs in their center. The particles that the scientists produced for this study are covered with a peptide called AP2, which has been shown in past work to help nanoparticles traverse the blood-cerebrum hindrance. However, without exact models, it was hard to concentrate on how the peptides assisted with transport across veins and into cancer cells.
When the specialists conveyed these nanoparticles to tissue models of both glioblastoma and solid brain tissue, they found that the particles covered with the AP2 peptide were greatly improved at infiltrating the vessels encompassing the growth. They likewise showed that the vehicle happened because of restricting a receptor called LRP1, which is more plentiful close to cancers than in typical cerebrovascular vessels.
The specialists then filled the particles with cisplatin, a commonly utilized chemotherapy drug. At the point when these particles were covered with the focusing peptide, they had the option to really kill glioblastoma cancer cells in the tissue model. Nonetheless, particles that didn’t have the peptides wound up harming the sound veins as opposed to focusing on the growth.
“We saw expanded cell passing in growths that were treated with the peptide-covered nanoparticle contrasted with the exposed nanoparticles or free medication.” “Those covered particles showed greater explicitness of killing the growth, as opposed to killing everything in a vague manner,” Hajal says.
More successful particles
The scientists then had a go at conveying the nanoparticles to mice, utilizing a particularly careful magnifying instrument to follow the nanoparticles traveling through the mind. They found that the particles’ capacity to cross the blood-cerebrum obstruction was basically the same as what they had found in their human tissue model.
They likewise showed that covered nanoparticles conveying cisplatin could dial back cancer development in mice, but the impact wasn’t serious enough for what they found in the tissue model. The specialists say that this may be on the grounds that the cancers were in a further developed stage. They currently wish to test different medications, delivered by an assortment of nanoparticles, to see which could make the best difference. They also intend to apply their method to the treatment of various types of brain cancer.
“This is a model that we could use to plan more viable nanoparticles,” Straehla says. “We’ve just tried one kind of mind cancer, yet we truly need to extend and test this with a great number of others, particularly intriguing growths that are challenging to review since there may not be as many examples accessible.”
The researchers described the method they used to create the cerebrum tissue model in a new Nature Protocols paper, so that other labs can use it as well.