A global team of researchers led by the University of Liverpool has created primary films of a key protein involved in a natural pathway of ozone-harming substance formation, providing new insight into its reactant action.
A significant supporter of an unnatural weather change is the ozone-harming substance nitrous oxide, which is multiple times more harmful to the ozone layer than carbon dioxide. Nitrous oxide is a result of the denitrification pathway, which happens when unique sorts of miniature creatures eliminate overabundances of nitrate or nitrite from environments and convert them back to nitrogen gas.
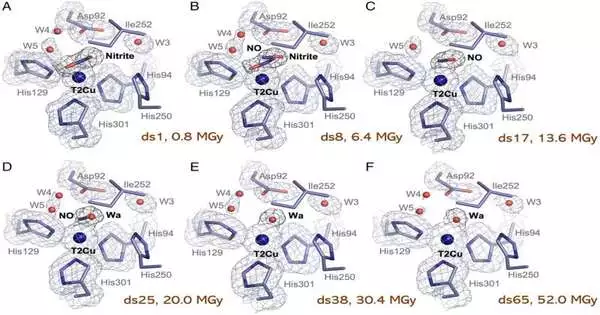
The initial step of this cycle includes a protein called copper nitrite reductase (CuNiR), which changes nitrite over completely to nitric oxide gas, utilizing an electron and a proton. As of late, a CuNiR from a Rhizobia animal type has been found with a considerably lower reactant action. This species is bountiful in farming and is a significant supporter of the denitrification pathway and hence nitrous oxide.
“For two reasons, the research is significant. First off, it aids in our comprehension of why this CuNiR’s activity is lower than that of others, which can be useful for upcoming bioengineering efforts to combat global warming. Furthermore, it demonstrates how intriguing a combination the MSOX method and single crystal spectroscopy is for analyzing complex redox processes in other fundamental metalloenzymes.”
Samuel Rose
CuNiR is a metalloprotein, meaning it contains metal particles to work accurately. In this situation, it contains two copper locales, one where catalysis happens and one more which gets and gives an electron required for catalysis. Metalloproteins are broad in science, making up no less than 30% of all proteins.
Analysts from the U.K. What’s more, Japan utilized single gem spectroscopy and an X-beam crystallography approach known as MSOX (various designs from one gem) to create a sub-atomic film of the protein to comprehend why the action is a lot lower in this CuNiR. X-beam crystallography is a significant method that permits the nuclear subtleties of natural atoms to be imagined in three aspects, assisting with understanding how they are gathered, how they capability, and how they connect. MSOX is a step forward on this as it permits catalysis to be pictured continuously.
First creator, Ph.D. understudy Samuel Rose, said, “This exploration is significant for two reasons. In the first place, it assists us with understanding why the action in this CuNiR is lower compared with others, which can assist with future bioengineering to assist with handling an unnatural weather change. Also, it shows that the MSOX approach along with single gem spectroscopy is a thrilling mix that can assist with taking apart complex redox responses in other key metalloenzymes. “
Professor Samar Hasnain, who drove the examination at the University of Liverpool, said, “It is simply by understanding key organic and compound cycles that we will actually want to handle major natural issues. The methodology created for this study would be material to numerous frameworks incorporating those engaged with hydrogen creation (hydrogenase), nitrogen use (nitrogenases), and photosynthesis (Photosystem II). “
The exploration is distributed in the Proceedings of the National Academy of Sciences.
More information: Samuel L. Rose et al, Single crystal spectroscopy and multiple structures from one crystal (MSOX) define catalysis in copper nitrite reductases, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2205664119
Journal information: Proceedings of the National Academy of Sciences