Public University of Singapore physicists have found how acetic acid derivation esters could be electro-blended from water and carbon monoxide in an economically feasible way.
The electrocatalytic decrease of carbon dioxide or carbon monoxide utilizing sustainable power is a promising green strategy for the creation of synthetics. So far, many carbonaceous items, including alcohols, hydrocarbons, and carboxylic acids, have been made utilizing this strategy. Strangely, esters, a significant group of natural mixtures, have not been shaped similarly. There are many purposes for esters, some of which incorporate solvents and oils. They are traditionally created through stoichiometric reactions like Fischer esterification. The beginning reagents are normally gotten from fossil assets, and compound waste is created after the response, which completes the cycle of earth hostile.
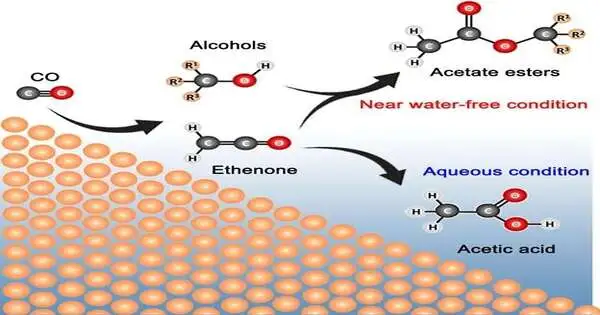
An exploration group driven by Associate Professor Yeo Boon Siang (Jason) from the Department of Chemistry, NUS, as a team with Dr. Sumit Verma and Dr. Ramesha Ganganahalli from the global energy organization Shell, has found how C3—C6 acetic acid derivation esters can be electro-blended from carbon monoxide and water, involving copper impetuses in a film cathode gathering. Ethyl acetic acid derivation and propyl acetic acid derivation can be created with an all-out Faradaic proficiency (FE) of around 22% and with an ongoing thickness of up to 55 mA/cm2, supported by minor amounts of methyl acetic acid derivation and butyl acetic acid derivation. The esters were created through the expansion response of ethenone (H2C=C=O) and alcohols delivered during CO decrease. The close to sans water response condition and the high nearby pH level play key parts in the development of the esters.
“This effort is the outcome of a collaboration between NUS and Shell to generate chemicals in a sustainable manner. Our team has developed an electrochemically driven technique for producing esters using renewable feedstocks such as carbon monoxide and water.”
Associate Professor Yeo Boon Siang
NUS and Shell had agreed before last year to mutually foster cycles to make green fills and synthetics for the energy business.
Prof Yeo said, “This work is a consequence of the cooperation between NUS and Shell to reasonably make synthetics.” Our group has prevailed with regards to fostering an electrochemically-driven cycle to make esters utilizing sustainable feedstocks like carbon monoxide and water. “
Expanding on the examination discoveries from their work, the exploration group intends to foster impetuses with higher ester change proficiency.
More information: Yansong Zhou et al, Production of C 3 –C 6 Acetate Esters via CO Electroreduction in a Membrane Electrode Assembly Cell, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202202859
Journal information: Angewandte Chemie International Edition