Epoxides are a type of chemical compound known as “cyclic ether,” which is distinguished by a three-atom ring. They are easily accessible substances that can be found in natural products, agrochemicals, and pharmaceuticals.
Epoxides are a useful industrial precursor because, through a reductive ring-opening reaction, they enable the synthesis of a wide variety of significant alcohols, functional polymers, agrochemicals, and medicines. Titanocene(III) has been the representative and exclusive catalyst for the ring-opening reaction for the past 30 years.
Titanocene-catalyzed reactions, on the other hand, are regioselective, favoring some by-products over others. The preferable goods in this scenario are those made from more stable radicals (as opposed to less stable radicals). It is yet unknown what mechanism underlies this regioselectivity.
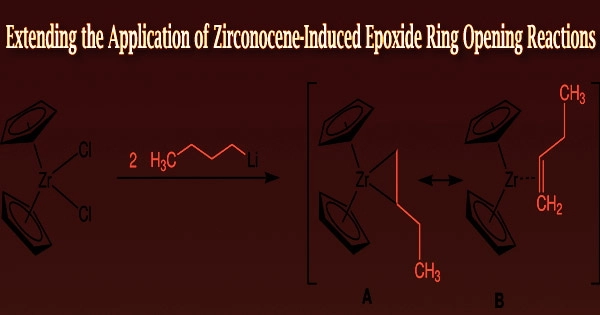
Researchers from the Department of Applied Chemistry at Waseda University in Japan, including Professor Junichiro Yamaguchi, Graduate Students Kazuhiro Aida, and Marina Hirao, and Assistant Professor Eisuke Ota, looked into zirconocene, the zirconium counterpart to titanocene, as a potential substitute catalyst for the ring-opening reaction.
“Zirconium is more oxophilic compared to titanium, which means it has a higher tendency to interact with oxygen atoms. This changes the pathway of the chemical reaction. We found that changing the titanium metal center to zirconium makes the ring-opening reaction more exothermic, which decreases the activation energy required for ring opening,” explains Ota.
Zirconium is one of the most abundant elements in Earth’s crust, which makes it readily available and low-cost. Since our reaction can be catalyzed using visible light, it is also environmentally friendly. With further improvement, our method could be a key contributor to green chemistry.
Professor Junichiro Yamaguchi
The team’s work revealed for the first time an epoxide ring opening catalysis based on zirconocene in the presence of only visible light. The researchers were encouraged by their results and went on to show their innovative method for a variety of substrates and functional groups, including natural materials.
However, the most intriguing feature of their strategy was that zirconocene-catalyzed reactions produced compounds with complimentary regioselectivity that was the opposite of that produced by titanocene-catalyzed reactions.
This made it possible to produce several elusive alcohol products that had previously been impossible to obtain due to regioselectivity problems. Zirconium also has an additional benefit over titanium.
As Yamaguchi explains:
“Zirconium is one of the most abundant elements in Earth’s crust, which makes it readily available and low-cost. Since our reaction can be catalyzed using visible light, it is also environmentally friendly. With further improvement, our method could be a key contributor to green chemistry.”
Overall, the findings of this work offer a fresh viewpoint that has the potential to greatly expand the range of reductive epoxide ring-opening reactions and add a fresh level to radical chemistry.