Water can be split up using electrocatalysis, which can be powered by renewable sources such as the sun or wind, to produce green hydrogen. A review article demonstrates how modern X-ray sources like BESSY II can help with the development of suitable electrocatalysts. X-ray absorption spectroscopy, in particular, can be used to determine the active states of catalytically active materials in the oxygen evolution reaction. This is a significant step toward creating efficient catalysts from inexpensive and widely available elements.
Green hydrogen is a viable energy carrier. It is created by electrolytically splitting water with wind or sun energy and storing this energy in chemical form. The electrodes are coated with catalytically active materials to facilitate the splitting of water molecules (and thus reduce the energy input). Dr. Marcel Risch and his Young Investigator Group Oxygen Evolution Mechanism Engineering are looking into the evolution of oxygen in water electrocatalysis. This is due to the fact that oxygen evolution, in particular, must be more efficient in order to produce economical hydrogen.
In order to produce green hydrogen, water can be split up via electrocatalysis, powered by renewable sources such as sun or wind. A review article in the journal Angewandte Chemie Int. Ed. shows how modern X-ray sources such as BESSY II can advance the development of suitable electrocatalysts. In particular, X-ray absorption spectroscopy can be used to determine the active states of catalytically active materials for the oxygen evolution reaction. This is an important contribution to developing efficient catalysts from inexpensive and widely available elements.
With X-ray absorption spectroscopy, we can not only determine the oxidation numbers, but also observe corrosion processes or phase changes in the material. Combined with electrochemical measurements, the measurement data thus provide a much better understanding of the material during electrocatalysis.
Dr. Marcel Risch
Exciting class of materials
An exciting class of materials for electrocatalysts are manganese oxides, which occur in many different structural variants. “A decisive criterion for suitability as an electrocatalyst is the oxidation number of the material and how it changes in the course of the reaction,” explains Risch. In the case of manganese oxides, there is also a great diversity in possible oxidation states. X-ray absorption spectroscopy (XAS) provides information about the oxidation states: X-ray quanta with suitable energy excite electrons on the innermost shells, which absorb these quanta. Depending on the oxidation number, this absorption can be observed at different excitation energies. Risch’s team has constructed an electrolysis cell that enables XAS measurements during electrolysis.
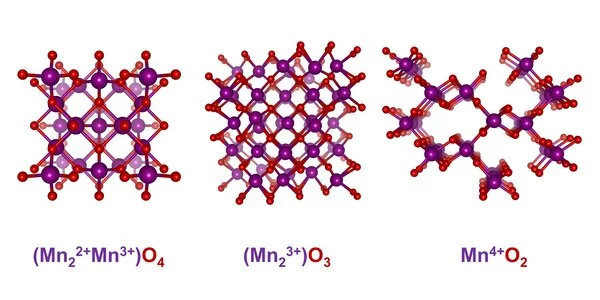
X-ray absorption spectroscopy
“With X-ray absorption spectroscopy, we can not only determine the oxidation numbers, but also observe corrosion processes or phase changes in the material,” says Risch. Combined with electrochemical measurements, the measurement data thus provide a much better understanding of the material during electrocatalysis. However, the required high intensity of the X-rays is only available at modern synchrotron light sources. In Berlin, HZB operates BESSY II for this purpose. There are about 50 such light sources for research worldwide.
Time scales from short to long
Risch still sees great potential for the application of X-ray absorption spectroscopy, especially with regard to the time scales of observation. This is because typical measurement times are a few minutes per measurement. Electrocatalytic reactions, however, take place on shorter time scales. “If we could watch electrocatalysis as it happens, we could better understand important details,” says Risch. With this knowledge, cheap and environmentally friendly catalysts could be developed more quickly.
On the other hand, many “ageing” processes take place within weeks or months. “We could, for example, examine the same sample again and again at regular intervals to understand these processes,” Risch advises. This would also make it possible to develop electrocatalysts with long term stability.





