Late examination has recognized MYT1 as a promising new helpful objective for bosom and gynecological malignant growth and found a progression of novel, intense, and profoundly particular inhibitors explicitly focusing on MYT1.
These discoveries, distributed in the Diary of Restorative Science, were upheld by Insilico Medication’s artificial intelligence-driven generative science and science motor.
Across the world, bosom and gynecological tumors present serious dangers to ladies’ wellbeing, fruitfulness, and, generally speaking, personal satisfaction. To distinguish expected focuses for new therapeutics, the examination group utilized Insilico’s restrictive artificial intelligence-driven target ID stage, PandaOmics, to break down information on five types of gynecological malignant growths, including ovarian, endometrial, cervical, and bosom disease, especially triple-negative bosom disease.
Strikingly, MYT1 is reliably positioned at the front across all illnesses with respect to importance.
“In addition to providing a strategy for successful target identification, this program’s innovative approach has resulted in the development of a promising selective MYT1 inhibitor, Compound 21, which broadens Insilico’s synthetic lethal pipeline and opens the door to a safer, more effective therapeutic future for patients battling breast and gynecological cancers.”
Said Yazhou Wang, Ph.D., medicinal chemistry leader of the MYT1 program from Insilico Medicine, and the first author of this paper.
MYT1 is an individual from the Wee1-kinase family, seldom communicated in most typical tissues but profoundly communicated in most malignant growth types. It has been accounted for that MYT1 hindrance and CCNE1 enhancement, a condition known as manufactured lethality, play urgent capabilities in cell cycle guidelines, which demonstrates MYT1 restraint is a promising engineered deadly remedial methodology for the therapy of diseases with genome precariousness (for example, CCNE1 intensification).
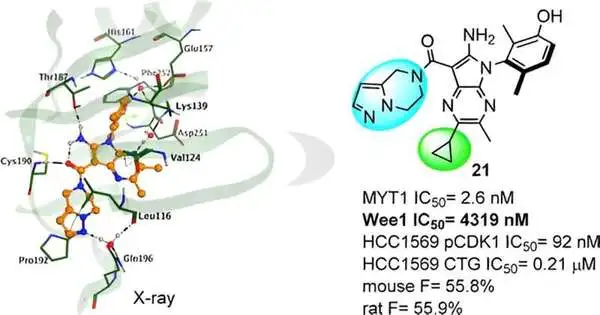
Nonetheless, MYT1 is profoundly homologous to Wee1, which makes it difficult to plan specific MYT1 inhibitors. In this review, Insilico tended to the hole, in particular MYT1 inhibitors, with the backing of Chemsitry42, Insilico’s simulated intelligence-driven little particle age stage.
Utilizing structure-based drug plan (SBDD) procedures and applying thorough channels for similitude and selectivity, Insilico planned a variety of mixtures focusing on MYT1 without any preparation. Among these original mixtures, one series arose as hit compounds.
Insilico then, at that point, directed an X-beam precious stone design examination of the complex and tracked down a huge effect on the action of unobtrusive compound construction changes. This information gave direction to additional sub-atomic streamlining, driving Insilico to the disclosure of the lead compound, Compound 21.
Compound 21 presents great MYT1 movement and magnificent selectivity over Wee1, and the other kinase board lessens the possible gamble for Askew impacts and could mean a more secure profile. In preclinical examinations, it likewise shows strong in vivo antitumor viability and a promising profile in ADME and PK/PD.
“The creative methodology of this program has not just introduced a strategy for compelling objective distinguishing proof; it has likewise prompted the improvement of a particular MYT1 inhibitor,” said Yazhou Wang, Ph.D., restorative science head of the MYT1 program from Insilico Medication and the primary creator of this paper. “Compound 21 grows Insilico’s manufactured deadly pipeline and prepares toward a more secure, more viable, and more helpful future for patients fighting gynecological and bosom diseases.”
More information: Yazhou Wang et al, Discovery of Tetrahydropyrazolopyrazine Derivatives as Potent and Selective MYT1 Inhibitors for the Treatment of Cancer, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.3c01476