A group of scientists from IBM Research Europe, Universidade de Santiago de Compostela and the University of Regensburg have changed the connections between the iotas in a solitary particle interestingly. In their paper, distributed in the journal Science, the gathering depicts their strategy and potential purposes for it. Igor Alabugin and Chaowei Hu have distributed a perspective piece in a similar diary issue framing the work done by the group.
According to Alagugin and Chaowei, the ongoing strategy for creating complex particles or subatomic gadgets is generally very testing — they compare it to unloading a case of Legos in a clothes washer and it is made to trust that a few helpful associations will emerge.In this new effort, the exploration group has made such work considerably simpler by utilizing a scanning burrowing magnifying lens (STM) to break the bonds in a particle and then to redo the atom by making new bonds—a science first.
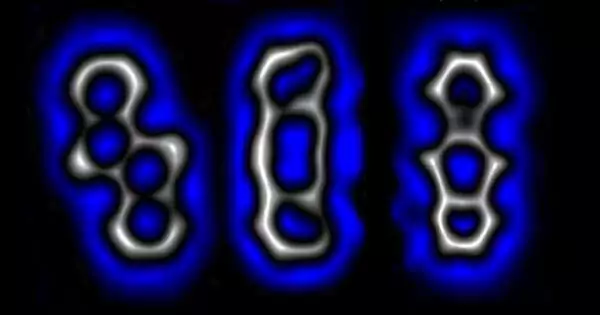
The schematic of the tip prompted responses. By voltage beats from the tip of a checking test magnifying lens, different sub-atomic changes are set off specifically. The shade of the bolts shows the amount of voltage beats used to set off the various changes.
The work by the group included putting an example material into a checking burrowing magnifying lens and, afterward, utilizing a small measure of power to break explicit bonds. More explicitly, they started by pulling four chlorine iotas from the center of a tetracyclic to use as their beginning particle. They then moved the tip of the STM to a C-CI bond and, afterward, broke the bond with a shock of power. Doing as such to the next C-CI and C matches brought about the development of a diradical, which left six electrons free for use in framing different bonds. In one trial of making another particle, the group then utilized the free electrons (and a portion of high voltage) to shape slanting C-bonds, bringing about the formation of a bowed alkyne. In another model, they applied a portion of low voltage to make a cyclobutadiene ring.
The scientists note that their work was made conceivable by the improvement of ultrahigh accuracy burrowing innovation created by a group headed by Gerd Binnig and Heinrich Rohrer, both at IBM’s lab in Zurich. They propose that their method could be utilized to more readily grasp redox science and to make new sorts of atoms.
More information: Florian Albrecht et al, Selectivity in single-molecule reactions by tip-induced redox chemistry, Science (2022). DOI: 10.1126/science.abo6471