Renewable energy sources like solar and wind will overtake conventional power grids in the ensuing decades. Since those sources only produce electricity when it’s sunny or windy, a reliable grid—one that can supply power around the clock—requires a way to store electricity when supplies are plentiful and deliver it later when they aren’t. For example, there may be periods of time when there is no wind, so some energy storage devices need to be able to store a significant amount of electricity for a long period of time.
The flow battery, an electrochemical device that can store hundreds of megawatt-hours of energy—enough to keep thousands of homes running for many hours on a single charge—is a promising technology for accomplishing that task. Flow batteries’ novel design contributes to their potential for long lifespans and low costs. The materials that store the electric charge in typical batteries are solid coatings on the electrodes found in cell phones and electric vehicles.
According to Fikile Brushett, an associate professor of chemical engineering at MIT, “a flow battery takes those solid-state charge-storage materials, dissolves them in electrolyte solutions, and then pumps the solutions through the electrodes.” Both advantages and difficulties come with that design.
“These solid-state charge-storage components are dissolved in electrolyte solutions and then pumped across the electrodes in a flow battery.”
Fikile Brushett, an associate professor of chemical engineering at MIT.
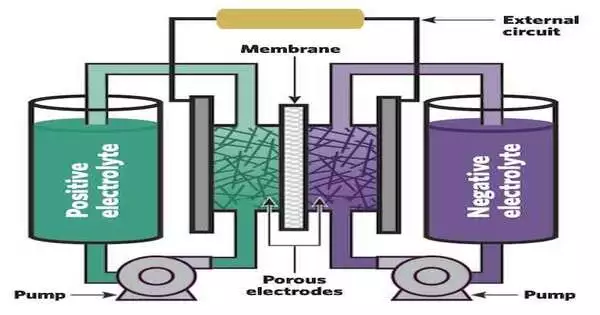
Flow batteries: construction and use
In a flow battery, two materials interact electrochemically, transferring electrons from one to the other. The two materials are forced into a state that is “less energetically favorable” when the battery is being charged because it stores more energy. (Consider a ball being propelled to the top of a hill. As the stored energy is released during battery discharge, the transfer of electrons moves the materials into a more energetically advantageous state. The player releases the ball and lets it roll down the slope.
A flow battery is composed of two sizable tanks, one holding positive and the other holding negative liquid electrolytes. Every electrolyte contains dispersed “active species”—atoms or molecules that will electrochemically react to release or store electrons. One species “oxidizes” (releases electrons) while the other is “reduced” (gains electrons) during charging; they switch positions during discharging. The two electrolytes are circulated by pumps through separate electrodes made of porous materials that offer lots of surfaces for the active species to react on. A thin membrane separating the electrodes keeps the two electrolytes from making direct contact and potentially reacting. If this happened, heat would be released, and energy that could have been used on the grid would be wasted.
As the battery is discharged, active species on the negative side oxidize, releasing electrons that travel through an external circuit to the positive side, where they reduce the species there. The grid can be powered by the flow of those electrons through the external circuit. To help complete the reaction and maintain the system’s electrical neutrality, “supporting” ions—other charged species in the electrolyte—pass through the membrane in addition to the movement of the electrons.
The system can be recharged once all the species have responded and the battery has been completely depleted. The reverse reactions in that process are propelled by electricity from wind turbines, solar farms, and other generating sources. The positive side’s active species oxidize, releasing electrons that travel back through the wires to the negative side and rejoin the original active species there. Now that it has been reset, the battery is prepared to supply more power as required. The battery can be cycled in this manner endlessly for years, continues Brushett.
both advantages and difficulties.
The fact that the energy storage in this system (the tanks) is distinct from the electrochemical reactions (which take place in the so-called reactor, which consists of porous electrodes and membranes), is a significant advantage. Because of this, the battery’s power and capacity—the amount of energy it can store and the rate at which it can be charged and discharged—can be adjusted independently. The tanks can be increased in size if Kara Rodby Ph. decides she needs more capacity. D. ‘ 22), a former student in Brushett’s lab, who is now employed by Volta Energy Technologies as a technical analyst. Additionally, the reactor’s size can be increased if I want to increase its power. Because of its adaptability, a flow battery can be created to suit a specific application and modified down the road if necessary.
But over time and with use, the electrolyte in a flow battery can deteriorate. All batteries experience electrolyte degradation, but flow batteries in particular experience “crossover,” a form of degradation that happens relatively quickly. The membrane is intended to let small supporting ions through while blocking the larger active species, but in practice it isn’t completely selective. The electrolyte in one tank can mix with some of the active species in the other tank if they “cross over” (or sneak through). The battery could then be discharged as a result of a chemical reaction between the two active species. Even if they don’t, some of the active species are no longer in the first tank, where they belong, which reduces the battery’s overall capacity.
Remediation is necessary to restore the capacity that crossover has reduced, such as by changing the electrolyte in one or both tanks or finding a way to restore the “oxidation states” of the active species in the two tanks. (An atom’s or compound’s oxidation state is indicated by a numerical value that indicates how many more or fewer electrons it has than when it is in its neutral state.) Because all the components are easier to access in a flow battery than they are in a conventional battery, such remediation is more easily—and consequently more cost-effectively—executed in a flow battery.
Vanadium, which is cutting-edge.
The chemistry chosen is an important consideration when designing flow batteries. Although the two electrolytes can contain various substances, the most popular configuration at the moment uses vanadium on both sides in various oxidation states. The two primary problems with flow batteries are resolved by that arrangement.
First off, vanadium doesn’t deteriorate. According to Brushett, “if you put 100 grams of vanadium into your battery and you come back in 100 years, you should be able to recover 100 grams of that vanadium—as long as the battery doesn’t have some sort of physical leak.”.
Second, if some vanadium from one tank crosses the membrane to the other, there won’t be any long-term contamination of the electrolytes; rather, there will only be a shift in the oxidation states, which can be quickly fixed by re-balancing the electrolyte volumes and reversing the shift with a quick charge step. The majority of modern commercial systems have a pipe that connects the two vanadium tanks and, when the tanks become out of balance, automatically transfers a certain amount of electrolyte from one tank to the other.
However, more and more flow batteries will be required to provide long-duration storage as the grid is replaced by renewable energy sources. There will be an issue as the demand for vanadium increases. According to Rodby, vanadium is present on every continent, but it is only found in trace amounts, making extraction challenging. “As a result, it is produced in a small number of locations, primarily in Russia, China, and South Africa, and the supply chain is unreliable. As a result, vanadium prices are high and very volatile, which hinders the widespread use of the vanadium flow battery.
In addition to vanadium.
Then the question is: If not vanadium, then what? Researchers from all over the world are attempting to answer that question, and many are concentrating on promising chemistries using materials that are more accessible and less expensive than vanadium. But Rodney says it’s not that simple. Other chemicals might have lower initial investment costs, but they might have higher operating costs. To restore one or both of their electrolytes, they might need routine maintenance. “You might even have to replace them, so you’re basically incurring that initial (low) capital cost repeatedly,” says Rodby.
Because there are “so many dependent variables,” as Brushett puts it, it is challenging to compare the economics of various options. “A flow battery is an electrochemical system, so it requires cooperation between a number of different parts in order to operate. Because of this, it is very difficult to improve a system in any way—performance, cost, whatever—because every time you change one thing, five other things change as well.
So how do we meaningfully compare these new and emerging chemistries with today’s vanadium systems, and how do we compare them with one another so we know which ones are more promising and what the potential pitfalls are with each one? “Addressing those questions can help us decide where to focus our research and where to invest our research and development dollars now,” says Brushett.
As a guide, use techno-economic modeling.
Techno-economic modeling is a useful tool for comprehending and evaluating the economic viability of new and emerging energy technologies. With certain models, it is possible to take into account the initial investment in a defined system as well as the ongoing operating expenses based on the system’s anticipated performance, yielding a total cost that has been discounted over the system’s lifetime. This outcome enables an option comparison on a “levelized cost of storage” basis for potential buyers.
Rodby created a framework for calculating the levelized cost of flow batteries using that method. The framework incorporates a physical battery model that is dynamic and tracks the battery’s performance over time, including any alterations in storage capacity. Therefore, all services required over decades of operation are included in the calculated operating costs, including the corrective actions taken in response to species degradation and crossover.
Since it would be impossible to analyze every possible chemistry, the researchers concentrated on specific classes. They started by eliminating possibilities and focusing on those in which the active species are dissolved in water. According to Rodby, aqueous systems are the most developed and have the greatest chance of being profitable. Then, they restricted their analyses to “asymmetric” chemistries, or set-ups where various materials are used in the two tanks. (As Brushett notes, vanadium is unusual in that it is infrequently possible to use the same “parent” material in both tanks.) Finally, they separated the potentials into two categories: species with a finite lifetime and species with an infinite lifetime, or, in other words, species that deteriorate over time and species that don’t.
They haven’t found any chemistry that stands out in the results of their analyses as being particularly effective. However, they do offer broad guidelines for picking and pursuing the various options.
Materials with a finite lifespan.
Unlike the finite-life materials, which are typically organic molecules made up of several different elements, including carbon, vanadium is a single element. The ability to be produced in a lab and on a large scale, as well as the ability to modify their structure to perform a particular function, are some advantages of organic molecules. The molecule could, for instance, be made larger so that it won’t fit through the membrane and cross to the other side, or it could be made more soluble so that more of it will be present in the electrolyte and the system’s energy density will be higher. Finally, simple, abundant, inexpensive elements, and perhaps even waste streams from other industries, can be used to create organic molecules.
Despite these appealing qualities, there are two issues. Organic molecules would probably need to be created in a chemical plant first, and upgrading the inexpensive precursors as necessary may end up costing more than intended. Second, these molecules are prone to degradation because they have substantial chemical structures that aren’t always very stable. As a result, Rodby adds, “you now have a new degradation mechanism that occurs over time, along with crossover. Furthermore, you might understand the process of degradation and how to stop it in one type of organic molecule, but the process might be completely different in the next molecule you work on, necessitating a significant amount of work in the discovery and development of each new chemistry.
Although research is ongoing, Rodby and Brushett currently find it difficult to defend the finite-lifetime chemistries, primarily because of their high capital costs. Rodby asserts that the current options cannot be produced at prices low enough to be commercially viable, citing studies that have calculated the manufacturing costs of these materials. They’re less expensive than vanadium, but not sufficiently so, according to Rodby.
The findings convey a crucial message to scientists developing novel organic chemistry: always take operating challenges into account at an early stage. According to Rodby and Brushett, practical concerns about a system’s long-term performance are frequently not addressed until much later on in the “innovation pipeline.”. The MIT team suggests that one of the initial design criteria should be to comprehend potential decay mechanisms and how they might be reasonably reversed or remedied.
Species with endless lifespans.
Materials with an infinite lifespan include vanadium and other non-degradable substances. Other metals, like iron or manganese, are the most likely contenders. These chemicals will undoubtedly be inexpensive because they are commodities, according to Rodby.
In this case, the researchers discovered that there is a larger “design space” of practical alternatives that could rival vanadium. However, there are still issues to be resolved. Although these species don’t break down, using them in a battery could result in unintended side effects. For instance, many metals catalyze the synthesis of hydrogen, which lowers efficiency and introduces a new type of capacity loss. Although there are solutions to the hydrogen evolution issue, a sufficiently inexpensive and successful solution for high rates of this side reaction is still required.
Furthermore, crossover continues to be an issue that needs to be fixed. Two approaches to preventing crossover in systems combining two different infinite-lifetime species types were evaluated by the researchers.
The “spectator strategy” is the first. In this location, both active species are present in both tanks. According to Brushett, “You have the same electrolyte mixture on both sides of the battery, but only one of the species is ever working and the other is a spectator. Crossover can therefore be handled in a manner akin to that of the vanadium-flow battery. The disadvantage is that each tank’s active material wastes away because only half of it can be used to store charge. According to Rodby, you have effectively doubled your electrolyte cost per unit of energy.
In order to maintain the electrical balance between the two sides using the second method, a perfectly selective membrane must be created. However, that strategy raises cell resistance, decreasing system effectiveness. Additionally, based on current production scales and methods, the membrane would need to be made of a special substance, like a ceramic composite, which would be very expensive. However, the cost and performance metrics are “far off from where they’d need to be to make sense,” according to Rodby, who notes that work on such membranes is currently underway.
The clock is ticking.
The urgency of the threat posed by climate change is emphasized, as is the requirement for grid-scale, long-duration storage systems to be available. There are currently many chemistries under consideration, but according to Rodby, we must focus on a few that will actually be able to compete with vanadium, can be implemented quickly, and can be used in long-term operations.”.
The goal of the techno-economic framework is to assist in directing this procedure. For comparison with vanadium systems and other designs, it can determine the levelized cost of storage for a given design. It can point out significant knowledge gaps related to long-term operation or remediation, thereby indicating which technological advancements or experimental studies should be given top priority. Furthermore, it can help determine whether, in these next-generation chemistries, the trade-off between lower upfront costs and higher operating costs makes sense.
The good news is that improvements made in research on one kind of flow battery chemistry can frequently be applied to others, as noted by Rodby. She claims that there are many concepts that can be applied to other systems. She thinks that the field has made progress in terms of knowledge as well as the capacity to create experiments that tackle issues shared by all flow batteries, assisting in laying the groundwork for the technology’s significant future role as grid-scale storage.
Provided by Massachusetts Institute of Technology