Discharges of ozone depleting substances contribute fundamentally to a dangerous atmospheric development. Furthermore, fluorine-containing gases, such as per- or polyfluorinated hydrocarbons, or PFCs, have a lot to offer in this turn of events.Specialists at the Institute of Organic Chemistry of Heidelberg University, driven by Prof. Dr. Michael Mastalerz, have recently grown new translucent materials that can specifically adsorb the atoms of such carbon-fluorine bonds. The Heidelberg specialists trust that these permeable precious stones might be valuable for designated restricting and recuperation of PFCs.
Polyfluorinated carbons are natural mixtures of different lengths in which the hydrogen particles of alkanes are somewhat or completely supplanted by fluorine iotas. These iotas are synthetically exceptionally steady. They are not universal in nature and are utilized primarily for carving processes in the semiconductor business, in eye medical procedures, and in clinical diagnostics as differentiation enhancers for specific ultrasound assessments.
“Not at all like CO2, which is coordinated in regular material cycles, PFCs gather in the climate and remain there for a few millennia prior to separating,” says Prof. Mastalerz. For all intents and purposes, PFCs have a lot more noteworthy Earth-wide temperature boost potential than carbon dioxide. The effect of one PFC particle is for all intents and purposes equivalent to 5,000 to 10,000 CO2 particles. As per the specialist, that makes polyfluorinated hydrocarbons an extremely durable issue that isn’t simply adding to the unnatural weather change currently speeding up too.
“Unlike CO2, which is incorporated into natural material cycles, PFCs build in the atmosphere and remain there for thousands of years before decomposing.”
Prof. Mastalerz
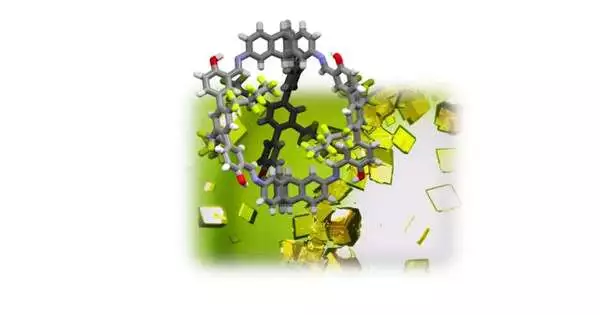
With his research group at the Institute of Organic Chemistry of Heidelberg University, Prof. Mastalerz has fostered another kind of translucent material that can adsorb polyfluorinated hydrocarbons profoundly, specifically, restricting them to its inside surface. The permeable precious stones depend on shape-tenacious natural enclosures that intensify and convey fluorine-containing side chains on the interconnected swaggers. These side chains respond as per the “like draws in like” rule through fluorine connections with the PFC atoms, guaranteeing they are kept on the internal surface of the material.
In their tests, the Heidelberg specialists demonstrated that the precious stones they created tie specific fluorine-containing gases, for example, octafluoropropane or octafluorocyclobutane, roughly 1,500 to multiple times more emphatically than dinitrogen, the fundamental part of air. As indicated by Prof. Mastalerz, these numbers address phenomenally high selectivities to tie such PFCs.
Presently, Prof. Mastalerz and his group are chipping away at increasing the selectivity of the gems and moving the cycle to other fluorinated gases, like those utilized in clinical sedation. “I see colossal potential for advancement around here,” says the scientist. He trusts that the adsorbent can be utilized for recuperation of polyfluorinated hydrocarbons at their place of purpose.
The examination results were distributed in the advanced materials.
More information: Ke Tian et al, Highly Selective Adsorption of Perfluorinated Greenhouse Gases by Porous Organic Cages, Advanced Materials (2022). DOI: 10.1002/adma.202202290
Journal information: Advanced Materials