Researchers from Johns Hopkins Medicine reveal unique alterations in the physiology of the “white matter” and other brain tissues in patients with post-treatment Lyme illness, a condition that affects 10% to 20% of the roughly half a million Americans who develop Lyme disease each year.
The study’s results, which were released on October 26, 2022, in the journal PLOS ONE, support and contribute to the validation of the idea that memory loss and other long-term cognitive problems experienced by people with post-treatment Lyme disease are related to functional and structural changes in the brain.
Lyme disease, whose early symptoms may include a characteristic rash, flu-like aches and fever, joint pain, and fatigue, is treated using a rigorous course of antibiotics, which usually clears the illness.
However, in those who are regarded as long-term patients and whose symptoms persist even after the use of antibiotics, Lyme disease is a chronic illness that can be accompanied by exhaustion, muscular pain, sleeplessness, depression, and cognitive challenges like memory loss and attention problems. These people typically lack overt clinical or laboratory evidence of persistent problems.
In a bid to identify what they suspect are long-term changes in brain function that may be causing certain persistent Lyme disease symptoms, researchers from Johns Hopkins Medicine’s departments of neurology and psychiatry and behavioral sciences, in collaboration with the Lyme Disease Research Center, used functional MRI (fMRI) scans of the brain, a technology that detects changes in brain blood flow. These scans allow investigators to track changes in the brain in real time.
Approaching this problem collaboratively with experts in different fields is what gave us these novel findings, and will hopefully answer our remaining questions, specifically about the role of axonal leakage and white matter activation in post-treatment Lyme disease.
“Objective biologic measures of post-treatment Lyme symptoms typically can’t be identified using regular MRIs, CT scans, or blood tests,” says researcher John Aucott, M.D., director of the Johns Hopkins Lyme Disease Clinical Research Center and associate professor of medicine at the Johns Hopkins University School of Medicine. “We needed to expand our methods of evaluation,” he adds.
Spearheaded by lead author Cherie Marvel, Ph.D., the team recruited 12 male and female post-treatment Lyme disease patients, and 18 participants without a history of Lyme, to undergo fMRI scans while performing a short-term memory task.
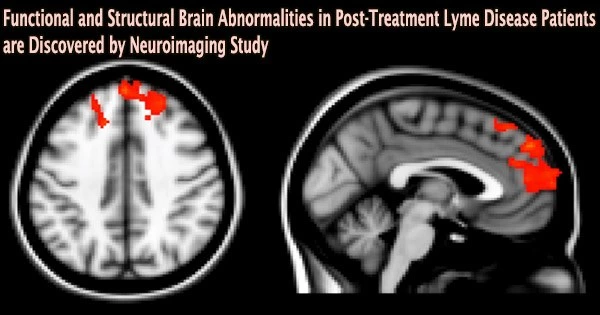
The frontal lobe, a part of the brain important for cognitive functions including memory recall and focus, showed atypical activity during the imaging tests. The alphabetical order of several letters as well as capital and lowercase letters had to be memorized and remembered by the participants.
Notably, the images revealed changes in frontal lobe activity between the two groups in the white matter of the brain, which is typically not seen in fMRI scans since this tissue functions with less blood flow than gray matter, according to the researchers. Similar to railroad tracks that assist bring information to the train depot, or gray matter, white matter is essential for transmitting information across the brain.
“We saw certain areas in the frontal lobe under-activating and others that were over-activating, which was somewhat expected,” says Marvel, an associate professor of neurology. “However, we didn’t see this same white matter activity in the group without post-treatment Lyme.”
To confirm this finding, researchers used a second form of imaging called diffusion tensor imaging (DTI) on all 12 participants with Lyme and 12 of the 18 non-Lyme participants. DTI detects the direction of water movement within brain tissue. This unique approach corroborated their fMRI findings and revealed new findings: Water was diffusing, or leaking, along the patients’ axons the extensions of neurons that carry electrical signals to other neurons within the same white matter regions identified in the fMRI.
Surprisingly, researchers discovered that among the post-treatment Lyme disease patients they evaluated, axonal leaking in white matter was associated with less cognitive abnormalities and better results.
As it corresponded with better disease outcomes, the researchers hypothesize that the enhanced activity they observed in white matter may reflect a beneficial immunological response in the post-treatment Lyme patients.
However, this finding was also correlated to the patients with post-treatment Lyme needing longer periods of time to complete the memory task. The researchers say this is indicative that the white matter response could be an adaptive one, but that the white matter’s physiology and function have been changed, which could come at a cost.
“Approaching this problem collaboratively with experts in different fields is what gave us these novel findings, and will hopefully answer our remaining questions, specifically about the role of axonal leakage and white matter activation in post-treatment Lyme disease,” she says.
Marvel and Aucott say this research may help illuminate underlying mechanisms and potential therapeutic targets for neurologic Lyme disease. Their findings may also have relevance to other kinds of infection-associated chronic illnesses, including long COVID and chronic fatigue syndrome.
Funding for the project was provided by an anonymous donor to John Aucott.
The research team also includes Kylie Alm, Deeya Bhattacharya, Alison Rebman, Arnold Bakker, Jason Creighton, Erica Kozero, Arun Venkatesan, and Prianca Nadkarni of Johns Hopkins Medicine; as well as Owen Morgan who is now at Cornell University. The authors declare no competing interests.