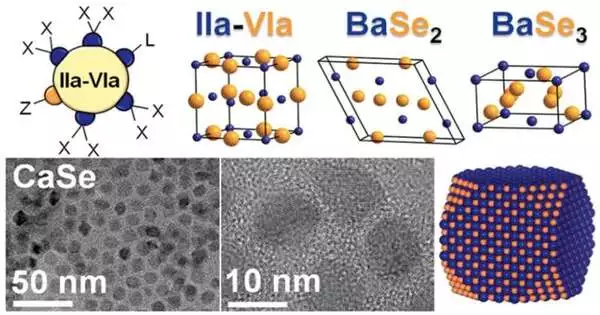
Investigation into the combination of new materials may result in more feasible and environmentally friendly items such as solar-powered chargers and light-emitting diodes (LEDs).Researchers from Ames Public Lab and Iowa State College have fostered a colloidal blend strategy for basic earth chalcogenides. This strategy permits them to control the size of the nanocrystals in the material. They were likewise ready to concentrate on the superficial level of science of the nanocrystals and survey the virtues and optical properties of the materials in question. Their exploration is examined in the paper “Basic Earth Chalcogenide Nanocrystals: Arrangement Stage Union, Surface Science, and Strength,” distributed in ACS Nano.
Basic earth chalcogenides are a kind of semiconductor that is of developing interest among researchers. They have various potential applications, for example, bioimaging, LEDs, and warm sensors. These mixtures may likewise be utilized to make optical materials, such as perovskites, which convert light into energy.
As per Javier Vela, Ames Lab researcher and the John D. Corbett Teacher of Science at Iowa State College, one reason these new materials are of interest is that “they involve earth-bountiful and biocompatible components, which make them good choices compared with the more widely utilized harmful or costly semiconductors.”
“They are made up of earth-abundant and biocompatible elements, making them preferable to other often used poisonous or expensive semiconductors.”
Javier Vela, Ames Lab scientist and the John D. Corbett Professor of Chemistry at Iowa State University,
Vela deduced that more widely used semiconductors contain lead or cadmium, both of which are harmful to human health and the environment.Also, the most famous method researchers use to blend these materials includes strong state responses. “These responses frequently occur at very high temperatures (over 900 °C or 1652 °F) and necessitate response times ranging from days to weeks,” he explained.
Then again, Vela made sense of that “arrangement stage (colloidal) science can be performed utilizing a lot of lower (under 300 °C or 572 °F) temperatures and more limited response times.” Thus, the colloidal strategy Vela’s group utilized requires less energy and time to blend the materials.
Vela’s group found that the colloidal blend strategy permitted them to control the size of the nanocrystals. Nanocrystal size is significant on the grounds that it decides the optical properties of certain materials. Vela made sense of the fact that by changing the size of the particles, researchers can impact how well the materials ingest light. “This implies we might possibly blend materials that are more appropriate for explicit applications by simply changing the nanocrystal size,” he said.
As per Vela, the group’s unique objective was to blend semiconducting basic earth chalcogenide perovskites due to their likely use in solar-powered gadgets. In any case, to achieve this objective, they required a more profound understanding of the key science of basic earth chalcogenides. They decided to zero in on these paired materials, all things considered.
Vela said that their examination fills a need to work on how researchers might interpret photovoltaic, glowing, and thermoelectric materials that are made of earth-plentiful and non-harmful components. “We trust that our advancements in this task will eventually guide in the union of more perplexing nanomaterials, for example, the basic earth chalcogenide perovskites,” he said.
Concentrate creators included Alison N. Roth, Yunhua Chen, Marquix A. S. Adamson, Eunbyeol Gi, Molly Wagner, Aaron J. Rossini, and Javier Vela.
More information: Alison N. Roth et al, Alkaline-Earth Chalcogenide Nanocrystals: Solution-Phase Synthesis, Surface Chemistry, and Stability, ACS Nano (2022). DOI: 10.1021/acsnano.2c02116
Journal information: ACS Nano