A study headed by Prof. Yuqin Zou (College of Chemistry and Chemical Engineering, Hunan University) and published in Science China Chemistry involved tests that were carried out utilizing a series of in situ characterization and density functional theory (DFT) calculations.
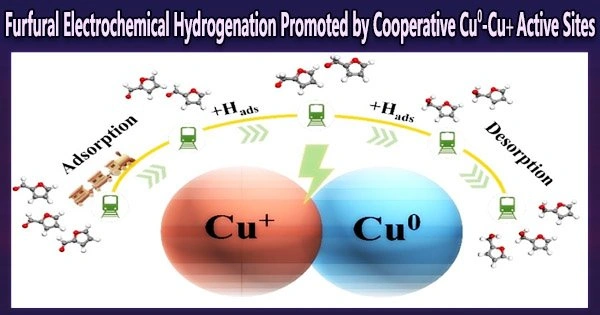
The experimental results highlight the slow hydrogenation rate and poor selectivity of Cu0-catalyzed furfural hydrogenation in the absence of Cu+. On the other hand, the Cu0-Cu+ active sites excel in selectively hydrogenating furfural to create furfuryl alcohol.
Moreover, this catalytic advantage exhibits a distinct potential dependence, with a consistent decline in furfural selective hydrogenation efficacy as the fraction of Cu+ declines with decreasing potential.
“Cu-based catalysts have shown excellent catalytic performance for the electrochemical hydrogenation of furfural to produce furfuryl alcohol. However, the true reaction active site remains unclear. Mixed-valence Cu oxide catalysts demonstrate excellent furfuryl alcohol selectivity but are limited by the dynamic electrocatalyst surface during catalysis. In situ capture of the true reaction activity sites and thus insight into the origin of the cu-based catalyst furfural electrochemical hydrogenation activity is necessary. This work could inform the optimal design of all Cu based catalyst electrocatalytic hydrogenation processes for organics,” Zou says.
Cu-based catalysts have shown excellent catalytic performance for the electrochemical hydrogenation of furfural to produce furfuryl alcohol. However, the true reaction active site remains unclear. Mixed-valence Cu oxide catalysts demonstrate excellent furfuryl alcohol selectivity but are limited by the dynamic electrocatalyst surface during catalysis. In situ capture of the true reaction activity sites and thus insight into the origin of the cu-based catalyst furfural electrochemical hydrogenation activity is necessary. This work could inform the optimal design of all Cu based catalyst electrocatalytic hydrogenation processes for organics.
Professor Yuqin Zou
Herein, the oxidation state of the prepared CuO nanowire under the ECH of furfural was tracked by in situ X-ray absorption spectroscopy (XAS). The co-existence of Cu0 and Cu+ states during the electrohydrogenation was con-firmed. Moreover, the poisoning experiment proved the decisive role of Cu+ in the furfural ECH.
Finally, the reaction energy barriers of the furfural ECH on Cu(111), Cu2O(111), and Cu0-Cu+ were analyzed by the density functional theory (DFT) calculation.
In conclusion, Cu0-Cu+ active sites on the surface of CuO synergistically convert furfural to furfuryl alcohol. The roles of Cu0 and Cu+ have also been revealed: Cu+ speeds up the second-step hydrogenation of furfural, and Cu0 lowers the energy barrier for desorption of furfuryl alcohol.