Researchers at the Fred Hutchinson Cancer Center in Seattle have just published two new studies that show how bacteria can infiltrate tumors and possibly aid in the growth and dissemination of cancers. The study team also demonstrated that the many microbes that make up a tumor’s microbiome may affect how a malignancy reacts to therapy.
The research also points to a connection between oral health and cancer because microorganisms in the mouth have been linked to tumors in other parts of the body.
The two papers one published Nov. 15 in Cell Reports and the other published Nov. 16 in Nature focus on an oral bacterium called Fusobacterium nucleatum, which has been linked to colorectal cancer.
Tumors often have help in their efforts to survive and grow. The presence of non-cancerous cells around a tumor can help it evade immune system attacks, withstand treatments that target them, and enable it to metastasize to other parts of the body. Researchers have now discovered that some of these helpful neighbors are actually bacteria rather than human cells.
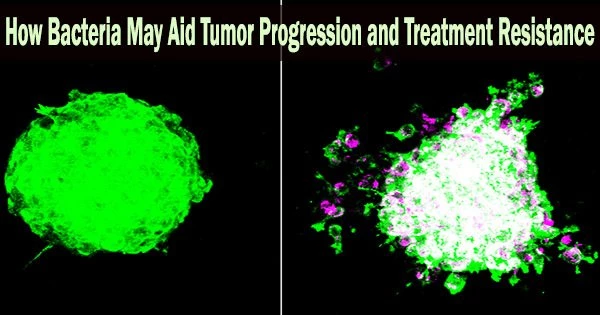
“What we’re showing is that there are regions of the tumor that are heavily colonized by bacteria micro-niche regions and they differ functionally from regions that do not harbor bacteria,” said Fred Hutch cancer microbiome researcher and study co-lead Susan Bullman, PhD, referring to the work outlined in the Nature study. “And these bacteria-rich regions have increased metastatic potential.”
Bullman and her collaborator, Fred Hutch molecular microbiologist Christopher D. Johnston, PhD, combined observations from tumors with lab-based experiments and small-molecule drug screens to show that F. nucleatum may shape conditions in tumors to keep them safe from immune attack and help them spread through the body. They found that some cancer therapeutics may be effective because they target both the bacteria that support the tumors as well as the tumor cells themselves.
The research team, which includes first author Jorge Galeano Niño a postdoctoral researcher at Fred Hutch, also found that other microbes including the intestinal bug Escherichia coli, or E. coli may render an anti-microbicidal and chemotherapeutic drug ineffective, which could shield both the tumor and F. nucleatum from treatment. These findings could help researchers develop new strategies to treat or target cancer by tackling its microbiome.
What we’re showing is that there are regions of the tumor that are heavily colonized by bacteria micro-niche regions and they differ functionally from regions that do not harbor bacteria. And these bacteria-rich regions have increased metastatic potential.
Susan Bullman
“This work is at the intersection of cancer and microbiome research,” Bullman said. “There’s compelling emerging data to suggest that nearly all major cancer types harbor an intra-tumoral microbiota.”
In colorectal cancer, an association with bacteria might make sense, but other cancers have also been found to harbor microbial communities, including breast, pancreatic, and lung tumors. Research is showing that tumor microbiomes may influence the development, progression, and response to treatment.
The scientists discovered that a variety of bacterial species were residing within oral and colorectal tumors, but they weren’t distributed uniformly, using a cutting-edge technique called spatial transcriptomics that enables researchers to determine where genes are turned on and off in slices of tumor tissue.
“We observed bacterial hotspots, or micro-niches, which opened up a number of questions as to how these formed and might be impacting cancer biology,” Johnston said.
Bacterially colonized areas showed a significantly suppressed immune system and fewer T cells that can fight cancer than other places. Immune checkpoint proteins, which limit the cancer-killing actions of T cells, were upregulated in areas where T cells were close to bacteria.
This study may shed light on how a patient’s microbiome may affect how well their cancer reacts to a checkpoint inhibitor. Several checkpoint medicines are licensed for treatment in colorectal cancer.
Specific findings from the studies:
Regions with bacteria were more likely to be necrotic dying with fewer dividing cells. Ironically, according to other research, this can be associated with metastasis because cells break off and travel to distant sites in the body.
In the lab, the researchers grew colorectal cancer spheroids, which are tumor cells grown in 3D clusters, with immune cells called neutrophils, which reduce T-cell migration and invasion. The neutrophils spread through spheroids without bacteria. But in those with bacteria, neutrophils migrated to the center of the spheroid and became trapped a finding that could explain why there are few T cells in regions colonized by bacteria.
Tumor cells in the spheroids moved differently when bacteria were present. Instead of moving in masses as a group, cancer epithelial cells migrated as single cells, bringing bacteria with them. This is consistent with Bullman’s previous work showing that F. nucleatum often hitches rides with colorectal cancer metastases.
Tumor cells infected with bacteria ramped up genes associated with cancer progression and metastasis. In oral tumor samples, the researchers saw that bacteria preferentially infected cancer epithelial cells and specific immune cells within patients’ tumors. Infected tumor cells had increased DNA damage signaling, a hallmark of cancer. These findings support bacteria having a direct role in shaping these micro-niche regions, the researchers said.
Some anti-cancer drugs may be effective because they are also antimicrobials that target bacteria supporting tumor development. The cancer-promoting bacterium F. nucleatum is highly sensitive to a common chemo drug called 5-fluorouracil, or 5-FU, but the researchers found that E. coli bacteria protected colorectal cancer cells from 5-FU. E. coli apparently has a way to metabolize the drug and minimize its exposure to cancer cells or other bacteria.
“The findings show that intra-tumoral microbes are not innocent bystanders during disease progression, and suggest that the microbiota should be taken into consideration when thinking about optimal cancer treatments,” Johnston said.
Related to these studies, the researchers are studying possible links between oral health and cancer risk.
“There is a trend emerging of microbes that are traditionally associated with oral inflammatory disease being found in association with extra-oral and gastrointestinal cancers which highlights the oral cavity as a breeding ground for pathogenic onco-microbes,” Johnston said.
“In addition to allowing pathogens to spread to new areas of the body, it is possible that inflammation in the mouth, in the form of periodontal or endodontic disease, could be selecting for and encouraging the outgrowth of bacteria that are more specialized for growth in adverse conditions and capable of evading immune attack,” he said.
The research team will continue to explore the possibility of making tumors more responsive to immunotherapy or chemotherapy by manipulating the microbiome, and they are seeking to design microbiome-modulating therapeutics that will prevent and treat cancer and stop its spread. Showing that microbes cluster in hard-to-reach areas of tumors, they have already clarified some of the hurdles they will have to overcome to develop these new approaches.
“This holistic approach to assessing the tumor microenvironment, which is a multi-species ecosystem, will advance our understanding of cancer biology, and I believe will reveal new therapeutic vulnerabilities in cancer,” Bullman said.
This work was supported by the National Institutes of Health, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, an Irvington Postdoctoral Fellowship from the Cancer Research Institute and a Washington Research Foundation Fellowship.