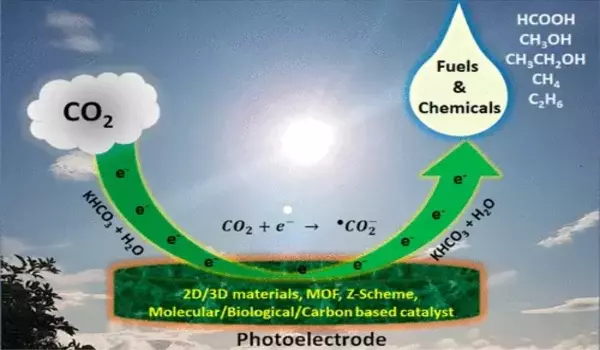
Copper catalysts have been shown to be effective in converting carbon dioxide (CO2) into useful liquid fuels such as methanol and ethanol. Recently, a team of researchers from the University of Toronto and the University of California, Berkeley developed a copper catalyst that sets a new record for efficiency in this process.
Researchers captured real-time video of copper nanoparticles evolving to convert carbon dioxide and water into renewable fuels and chemicals. Their new findings could aid in the development of the next generation of solar fuels.
Since the 1970s, scientists have known that copper has a unique ability to convert carbon dioxide into valuable chemicals and fuels. For many years, scientists have struggled to understand how this common metal works as an electrocatalyst, a mechanism that uses electron energy to chemically transform molecules into different products.
A research team led by Lawrence Berkeley National Laboratory (Berkeley Lab) has gained new insight by capturing real-time movies of copper nanoparticles (copper particles engineered at the scale of a billionth of a meter) converting CO2 and water into renewable fuels and chemicals such as ethylene, ethanol, and propanol. The findings were published in the journal Nature last week.
“This is extremely exciting. After decades of research, we can finally demonstrate – with undeniable evidence – how copper electrocatalysts excel at CO2 reduction” The study was led by Peidong Yang, a senior faculty scientist in Berkeley Lab’s Materials Sciences and Chemical Sciences Divisions. Yang is also a chemistry and materials science and engineering professor at UC Berkeley. “Knowing how copper is such an excellent electrocatalyst brings us one step closer to using artificial photosynthesis to convert CO2 into new, renewable solar fuels.”
Knowing how copper is such an excellent electrocatalyst brings us one step closer to using artificial photosynthesis to convert CO2 into new, renewable solar fuels.
Peidong Yang
To investigate the same sample environment: copper nanoparticles in liquid, the researchers combined a new imaging technique called operando 4D electrochemical liquid-cell STEM (scanning transmission electron microscopy) with a soft X-ray probe. The groundbreaking approach was conceived by Yao Yang, a UC Berkeley Miller postdoctoral fellow, while working toward his Ph.D. in chemistry at Cornell University under the supervision of Peidong Yang.
Scientists studying artificial photosynthesis materials and reactions have wished to combine the power of an electron probe with that of X-rays, but the two techniques are typically incompatible.
Electron microscopes (such as STEM or TEM) use electron beams to characterize the atomic structure of material parts. 4D STEM (or “2D raster of 2D diffraction patterns using scanning transmission electron microscopy”) instruments, such as those at Berkeley Lab’s Molecular Foundry, have pushed the boundaries of electron microscopy even further, allowing scientists to map out atomic or molecular regions in materials ranging from hard metallic glass to soft, flexible films.
Soft (or lower-energy) X-rays, on the other hand, are useful for identifying and tracking chemical reactions in real-time in an operando, or real-world, environment.
Scientists can now have the best of both worlds. A remarkable electrochemical “liquid cell” sample holder is at the heart of the new technique. The device, which is a thousand times thinner than human hair, is compatible with both STEM and X-ray instruments.
The ultrathin design of the electrochemical liquid cell allows for reliable imaging of delicate samples while protecting them from electron beam damage. The team was able to conduct X-ray experiments with the electrochemical liquid cell thanks to a special electrode custom-designed by co-author Cheng Wang, a staff scientist at Berkeley Lab’s Advanced Light Source. By combining the two, researchers can characterize electrochemical reactions in real-time and at the nanoscale.
Getting granular
Using the new electrochemical liquid cell, Yao Yang and colleagues observed copper nanoparticles (ranging in size from 7 nanometers to 18 nanometers) evolve into active nanograins during CO2 electrolysis – a process that uses electricity to drive a reaction on the surface of an electrocatalyst.
Copper nanoparticles combined into larger metallic copper “nanograins” within seconds of the electrochemical reaction, which surprised the researchers. To find out more, the researchers turned to Wang, who pioneered a technique known as “resonant soft X-ray scattering (RSoXS) for soft materials” at the Advanced Light Source over a decade ago.
The research team used the same electrochemical liquid cell, but this time during RSoXS experiments, with Wang’s assistance, to determine whether copper nanograins aid in CO2 reduction. Wang explained that soft X-rays are ideal for studying how copper electrocatalysts evolve during CO2 reduction. Researchers can use RSoXS to monitor thousands of nanoparticle reactions in real time and accurately identify chemical reactants and products.
The RSoXS experiments at the Advanced Light Source, along with additional evidence gathered at the Cornell High Energy Synchrotron Source (CHESS), demonstrated that metallic copper nanograins act as active CO2 reduction sites. (Metallic copper, also known as copper(0), is a type of copper.)
During CO2 electrolysis, the copper nanoparticles change their structure during a process called “electrochemical scrambling.” The copper nanoparticles’ surface layer of oxide degrades, creating open sites on the copper surface for CO2 molecules to attach, explained Peidong Yang. And as CO2 “docks” or binds to the copper nanograin surface, electrons are then transferred to CO2, causing a reaction that simultaneously produces ethylene, ethanol, and propanol along with other multicarbon products.
“The copper nanograins essentially turn into little chemical manufacturing factories,” Yao Yang said.
Experiments at the Molecular Foundry, Advanced Light Source, and CHESS revealed that size does matter. The 7-nanometer copper nanoparticles all reduced CO2, whereas the larger nanoparticles did not. Furthermore, the team discovered that only metallic copper can efficiently convert CO2 into multicarbon products. The results have implications for “rationally designing efficient CO2 electrocatalysts,” according to Peidong Yang.
The new study also validated Peidong Yang’s 2017 findings: That the 7-nanometer-sized copper nanoparticles require low energy inputs to begin reducing CO2. The 7-nanometer copper nanoparticles required a record-low driving force as an electrocatalyst, about 300 millivolts less than typical bulk copper electrocatalysts. The best-performing CO2 multicarbon catalysts typically operate at a high driving force of one volt.
Copper nanograins have the potential to improve the energy efficiency and productivity of some catalysts used in artificial photosynthesis, a field of study aimed at producing solar fuels from sunlight, water, and CO2. Researchers from the Department of Energy-funded Liquid Sunlight Alliance (LiSA) intend to use the copper nanograin catalysts in the design of future solar fuel devices.
“The ability of the technique to record real-time movies of a chemical process opens up exciting possibilities for research into many other electrochemical energy conversion processes. It’s a huge step forward, and it wouldn’t have been possible without Yao’s pioneering work” According to Peidong Yang.