Examining the exchange between the design of water particles that have been integrated into layered materials, for example, muds, and the setup of particles in such materials has long demonstrated an extraordinary technical challenge. However, scientists have now used a method that is normally used to gauge very small masses and sub-atomic connections at the nano level to notice these interesting collaborations.
Their exploration was distributed in Nature Correspondences.
At the nanoscale, many materials develop a layered structure.When dry, mud resembles a series of sheets stacked on top of one another.At the point when such layered materials experience water, nonetheless, that water can be bound and coordinated into the holes or openings—or, more precisely, the pores—between layers.
Such “hydration” can also occur when water particles or their constituent components, such as a hydroxide particle (an adversely charged particle composed of a single oxygen and a single hydrogen iota), are incorporated into the material’s glasslike structure.This sort of material, hydrate, isn’t really “wet,” despite the fact that water is currently important for it. Hydration can likewise considerably change the first material’s design and properties.
“In other words, the water structures are sensitive to the structure of the interlayer ions. And, whereas this ion arrangement determines how many ions may be stored in many different crystal structures, such configurations have rarely been carefully examined until now.”
Katsuya Teshima, corresponding author of the study
In this nanoconfinement, the hydration structures—how water particles or their constituent components organize themselves—decide the capacity of the first material to store particles (emphatically or adversely charged iotas or gatherings of molecules).
This water or charge capacity implies that such layered materials, ranging from regular muds to layered metal oxides—and, crucially, their connections with water—have a wide range of applications, ranging from water purification to energy capacity.
In any case, concentrating on the exchange between this hydration structure and the setup of particles in the particle stockpiling system of such layered materials has proven to be an extraordinary test. Also, endeavors at examining how these hydration structures shift over the direction of any development of these particles (particle transport) are much more troublesome.
Ongoing examination has shown that such water designs and connections with the layered materials assume a significant part in giving them their high particle stockpiling limits, all of which thus depend on how adaptable the layers that have the water are. In the space between layers, any pores that are not loaded up with particles get loaded up with water atoms, all things considered, assisting with settling the layered design.
“In other words, the water structures are sensitive to how the interlayer particles are organized,” said Katsuya Teshima, the review’s related writer and a materials physicist with Shinshu College’s Exploration Drive for Supra-Materials.”And keeping in mind that this particle setup in various gem structures controls the number of particles that can be stored, such designs have, as of recently, seldom been efficiently explored.”
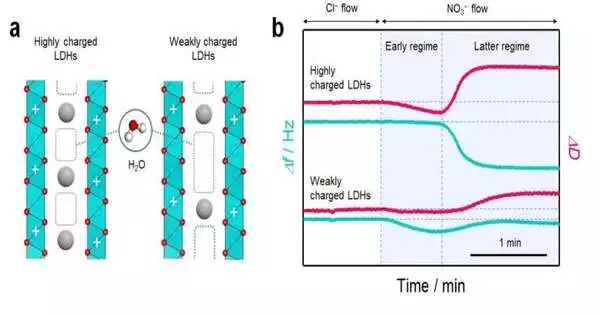
So Teshima’s gathering focused on quartz gem microbalance with energy scattering checking (QCM-D) to help with their hypothetical estimations. QCM-D is basically an instrument that works like an equilibrium scale and can gauge very small masses and sub-atomic connections at the nanoscale. The method can also detect small changes in energy loss.
The researchers used QCM-D to demonstrate that the construction of water atoms bound in the nano-space of layered materials can be tentatively observed.
They did this by estimating the “hardness” of the materials. They explored the layered twofold hydroxides (LDHs) of a class of adversely charged mud. They found that the hydration structures were related to the solidifying of the LDHs when any particle trade response occurred (a trading of one sort of particle with an alternate kind of particle yet with a similar change).
“As such, any adjustment of particle connection begins with the adjustment of the hydration structure that happens when particles are integrated into the nano-space,” added Tomohito Sudare, a partner on the project now with the College of Tokyo.
Also, the analysts found that the hydration structure is profoundly subject to the charge thickness (how much charge per unit of volume) of the layered material. This generally oversees the particle stockpiling limit.
The analysts currently desire to apply these estimation strategies along with the information on the hydration design of particles to devise new methods for further developing the particle stockpiling ability of layered materials, possibly opening new roads for particle division and feasible energy stockpiling.
More information: Tomohito Sudare et al, Critical role of water structure around interlayer ions for ion storage in layered double hydroxides, Nature Communications (2022). DOI: 10.1038/s41467-022-34124-9