The colors that you see when you look at a painting in a museum probably aren’t as bright as they were before, which was mostly due to exposure to light. A new cause of color deterioration has now been discovered by researchers: humidity.
“This new corruption pathway makes sense of why a few canvases’ tones might blur in any event when they’ve been put away in dim rooms,” Stanford Synchrotron Radiation Lightsource researcher Sam Webb said. “Understanding what causes many paintings of the Renaissance to change color or fade can help conservationists preserve our historical masterpieces.”
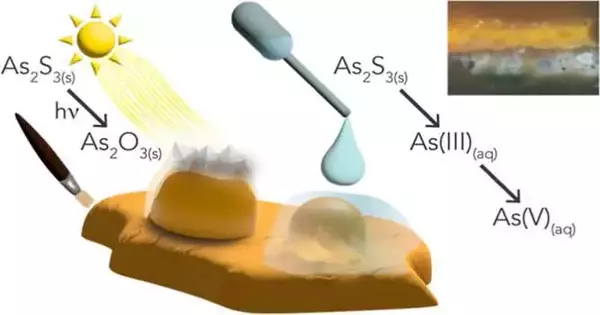
The researchers from SSRL at the Department of Energy’s SLAC National Accelerator Laboratory, the University of Amsterdam, and the Rijksmuseum, among other institutions, wanted to know why a yellow arsenic sulfide pigment called orpiment and an arsenic species called As(V) are found together. Past exploration has demonstrated the way that the transformation of the shades to As (V) can cause variety corruption; however, the underlying foundations of this change have remained muddled.
“This new degradation pathway explains why some paintings’ colors may fade even when they have been kept in dark rooms. Many paints of the Renaissance type can fade or change color, so understanding what causes these things to happen can help conservationists preserve our historical masterpieces.”
Stanford Synchrotron Radiation Lightsource scientist Sam Webb
To concentrate on the As(V) species, the exploration group removed a little example from the seventeenth-century painting “Still Existence with Blossoms in a Glass Jar,” by Jan Davidszoon de Heem. The painting’s central yellow eglantine rose has been turning white.
The researchers zapped the sample with bright X-rays from one of the beam lines at the SSRL to get a better look at the color shift in the yellow rose. These X-beams furnished specialists with 2D pictures of the example’s cross area and the areas of the arsenic species.
Johanna Nelson Weker, a scientist at the SSRL, stated, “The X-rays at the SSRL enabled us to get into the depth of the paint.” We saw underneath the surface layers, which have been presented to light, and into the layers that haven’t been corrupted by light.”
After taking X-ray images, the researchers put the paint sample under an X-ray microscope to get a higher-resolution and 3-D image of the arsenic species’ chemistry. The team was able to map the pathways of degradation thanks to X-rays that provided a two-dimensional, bird’s-eye view of the paint and close-up images of its three-dimensional structure.
According to Nelson Weker, these pathways demonstrated that the arsenic species travels on a trail made by humidity. The specialists distributed their discoveries in the Diary of the American Compound Society.
Also, they found that the arsenic species doesn’t remain exactly where the craftsman utilized orpiment shade. Conservators recently believed that corruption was gathered where the color was in a painting.
Webb stated, “This means that if you are a conservator, you must not only look where the arsenic pigment is, but also in a large halo around the pigment.”
Finding that dampness could assist with debasing varieties took a ton of experimentation, Webb said. In the beginning, the team completely recreated an arsenic pigment sample in the laboratory. Then, at that point, they added egg yolk—aa typical color cover utilized by craftsmen of the time—tto the example.
In the laboratory, they tested this egg-yolk sample under various humidity conditions and compared their findings to those of the sample from de Heem’s yellow eglantine rose. That uncovered that the arsenic species can spread in the wake of responding with water in lab-developed examples and in works of art that are many years old.
The team wants to learn more about how the pigments react with light, including from experimental X-rays, in the future. This will assist them with seeing every one of the responses between the shades and the covers in a more complicated setting and further assist in protection endeavors, Webb said. Also, the scientists might want to decide the exact scope of dampness levels that permit the arsenic species to fill in the shade.
More information: Fréderique T. H. Broers et al, Two Pathways for the Degradation of Orpiment Pigment (As2S3) Found in Paintings, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c12271