Using layers of hydrogels arranged into a cube-like structure, a team led by Masaya Hagiwara at the RIKEN National Science Institute in Japan has created an ingenious device that enables scientists to create intricate 3D organoids without the need for complex procedures. The team has also recently shown that they can use the tool to create organoids that accurately mimic the asymmetric genetic expression that distinguishes the actual development of organisms. The apparatus has the potential to transform drug testing as well as reveal details about how tissues grow and improve methods for growing artificial organs.
In an effort to mimic real biological development, scientists have long struggled to produce organoids—tissues that resemble organs grown in a lab. Since it’s crucial to comprehend how medicines move through various tissues, making organoids that behave like real tissues is crucial for developing new medications. Organoids are a first step toward developing complete organs that can benefit patients, and they also provide us with insights into the development process itself.
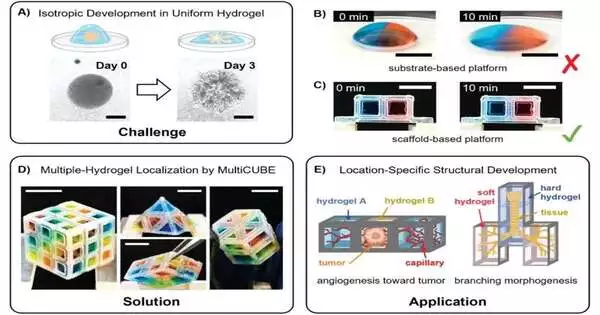
Organoids that resemble living things have proven challenging to make. Chemical gradients and physical scaffolds help direct cells into specific 3D patterns as tissues develop in nature through an intricate dance. Contrarily, in the laboratory, organoids are typically produced by either allowing cells to proliferate in homogeneous conditions, resulting in simple balls of similar cells, or by using 3D printing or microfluidic technologies, both of which require specialized tools and technical know-how.
The RIKEN Cluster for Pioneering Research team has now revealed the creation of a novel technique that enables them to spatially control the environment around clusters of cells based on cubes using nothing more complex than a pipette in an initial paper published in Advanced Materials Technologies.
The procedure entails layering hydrogels, which are primarily water-based substances with various physical and chemical characteristics, and containing them inside a cube-shaped culture vessel. The surface tension of the scaffold was used to hold various hydrogels in place as they were pipette-inserted into it for the study. A variety of tissue types could be created by inserting cells into the cubes, either within the individual hydrogels or as pellets that could move into the various layers.
In a second paper that was published in Communications Biology, the team also showed that it was possible to recreate so-called body-axis patterning. In essence, the patterning of cell differentiation during vertebrate development is head/rear and back/stomach. This has proven to be very challenging to accomplish in the lab, despite being crucial for the development of organoids that accurately mimic what occurs in real organisms.
In this study, a group of induced pluripotent stem cells (iPSCs) were precisely seeded inside a cube using the cube-based system, and the team was able to replicate this patterning by exposing the iPSCs to a gradient of two different growth factors. They even went so far as to successfully “recruit” a lab assistant and a junior high school student, demonstrating that the seeding of the cells wouldn’t require a high level of expertise. The group also showed that the tissues produced could be divided into sections for imaging while retaining the gradient orientation data.
According to Hagiwara, “We are very excited by these achievements, as the new system will allow researchers to quickly and without challenging technical hurdles recreate organoids that more closely resemble the way that organs develop in actual organisms. We hope that a variety of researchers will use our technique to produce various new organoids and contribute to the study of various organ systems. We anticipate that it will eventually aid in our understanding of how to create real artificial organs that benefit patients.”.
More information: Kasinan Suthiwanich et al, Localization of Multiple Hydrogels with MultiCUBE Platform Spatially Guides 3D Tissue Morphogenesis In Vitro, Advanced Materials Technologies (2023). DOI: 10.1002/admt.202201660
Isabel Koh et al, Gradient to sectioning CUBE workflow for the generation and imaging of organoids with localized differentiation, Communications Biology (2023). DOI: 10.1038/s42003-023-04694-5