Malaria has been a problem for people for thousands of years. Hippocrates, the “Father of Medicine,” mentioned the condition in a medical work around the 4th or 5th century BC. Even great warriors couldn’t stand a chance against the microscopic parasites, as Alexander the Great may have died of malaria at the age of 305. However, it wasn’t until 1718 that Italian physician Francisco Torti invented the term malaria, a title derived from the Roman physicians’ notion that the disease was named from tumors in the marsh air.
An international team has uncovered a protein that serves a critical biochemical role in the malaria parasite. Deactivating this protein inhibits the in vitro growth of Plasmodium falciparum, the protozoa responsible for the most severe form of the disease, by more than 75%. The findings were recently published in the scientific journal mBio by the team lead by Professor Dave Richard of Université Laval.
“This discovery could pave the way for the development of a malaria treatment that targets a function of the parasite that no malaria drug has yet exploited,” said Richard, a professor in the Faculty of Medicine at Université Laval and researcher at the CHU de Québec-Université Laval Research Centre.
The protein we discovered, PfPX1, is crucial in transporting hemoglobin to these digesting vacuoles. When we disable PfPX1, we deprive the parasite of its primary source of amino acids. This has an effect on its growth and survival. This discovery could pave the way for the development of a malaria treatment that targets a function of the parasite that no malaria drug has yet exploited.
Professor Richard
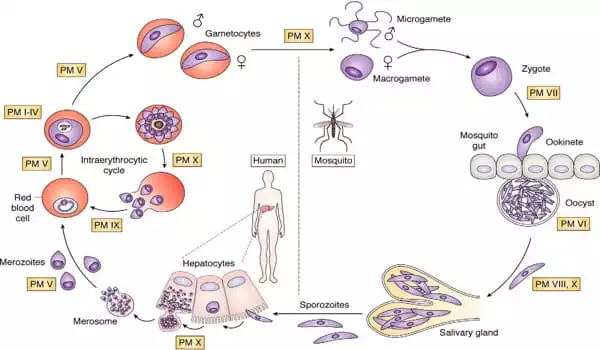
Plasmodium falciparum is transferred to humans through mosquito bites. After infecting the host’s liver, it circulates in the circulation, hiding inside red blood cells and therefore evading immune system responses. The parasite’s primary food supply is hemoglobin, the protein that transports oxygen from red blood cells to the rest of the body. The parasite digests the hemoglobin in structures known as digestive vacuoles.
“The protein we discovered, PfPX1, is crucial in transporting hemoglobin to these digesting vacuoles,” Professor Richard explained. “When we disable PfPX1, we deprive the parasite of its primary source of amino acids. This has an effect on its growth and survival.”
Richard sees a potential new strategy to fight malaria in light of these findings: “We were able to prevent the parasite’s PfPX1 protein from fulfilling its tasks. Because the protein isn’t found in people, there’s a lower chance of it interfering with any vital human activities.”
Malaria is a common, possibly dangerous disease with acute symptoms such as fever, headache, vomiting, and exhaustion. According to experts, 300-600 million people develop malaria each year, with more than one million dying. The majority of instances occur in Sub-Saharan Africa, and the majority of fatalities occur in children under the age of five. Although quinine and other anti-malarial drugs are available, patients who are not treated may experience illness recurrence months later.
Malaria is still a problem in many regions of the world, especially Sub-Saharan Africa. Malaria infected 241 million people in 2020, killing 627,000. Pregnant women and children under the age of five are more vulnerable to the sickness.
Despite the World Health Organization’s recognition of the first malaria vaccine last year, Richard believes it is critical to continue researching new therapeutic avenues: “As we’ve seen with COVID-19, new strains can emerge and jeopardize vaccine effectiveness. Furthermore, resistant strains of artemisinin, the major anti-parasite medicine used to treat malaria, have already evolved in Southeast Asia. It is critical to mix therapeutic methods, like we do with AIDS, to preserve treatment efficacy and limit the danger of emerging drug-resistant strains. Our research could play a role in the fight against malaria.”
Malaria parasites can be found in both humans and female Anopheles mosquitos. Because of the parasite’s size and genetic complexity, each infection exposes the human immune system to hundreds of antigens (proteins).
Even while in the human host, the parasite goes through numerous life stages, presenting various antigens at different phases of its life cycle. It has been difficult to determine which of these can be a suitable target for vaccine development. Furthermore, the parasite has devised a number of tactics to confuse, conceal, and misdirect the human immune system.