Many chemical processes require the use of a catalyst. It allows a molecule to transform into another without changing itself, allowing it to be reused several times. Many catalysts, on the other hand, are constructed of precious metals, making them costly and possibly hazardous to the environment.
Researchers have now developed a catalyst made of the far more abundant metal iron to aid in the olefin metathesis reaction, which is an important chemical reaction. Their findings were just reported in Nature Catalysis.
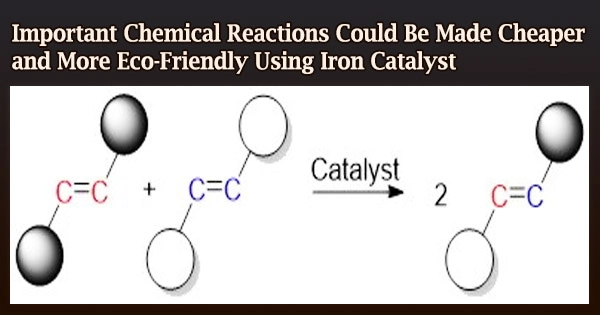
“The olefin metathesis reaction is among the most widely applicable catalytic reactions for carbon-carbon double bond formation,” explained Satoshi Takebayashi, a researcher at the Okinawa Institute of Science and Technology Graduate University (OIST) who was involved in the work. “Carbon-carbon double bonds are an important bond found in many chemical products.”
Olefins are a type of carbon-carbon double bonding chemical. By switching the carbon atoms in olefins, the olefin metathesis reaction creates new carbon-carbon double bonds. By breaking the initial double bonds and forcing new ones to form, the catalyst enables this swapping.
The valuable metal ruthenium is currently one of the most preferred catalysts for this reaction. The goal of this research was to speed up the reaction by employing a catalyst made of iron, a far more abundant metal, making the entire process less expensive and more environmentally benign.
This study can be useful to other researchers in the field. I hope that iron-based catalysts can be developed further using this knowledge.
Satoshi Takebayashi
Because ruthenium and iron are in the same group on the periodic table and are believed to have similar properties, this has been a long-sought goal in the scientific community.
The researchers created a novel iron complex and showed that it could be utilized as a catalyst in the olefin metathesis reaction in this study. They demonstrated its effectiveness by constructing a polymer, which is a long chain molecule made up of smaller chemical units.
Despite the success of this study, Takebayashi pointed out that current ruthenium-based catalysts are still far more useful than newly developed iron-based catalysts.
When exposed to air and moisture, the iron-catalyst becomes unstable and less active. Before the iron-catalyst can replace the ruthenium-catalyst, these constraints must be overcome.
“This study can be useful to other researchers in the field,” concluded Takebayashi. “I hope that iron-based catalysts can be developed further using this knowledge.”