The low-valent cationic aluminum complex [Al(AlCp*)3]+ has been successfully synthesized by the scientists Philipp Dabringhaus, Julie Willrett, and Prof. Dr. Ingo Krossing from the Institute of Inorganic and Analytical Chemistry at the University of Freiburg. The group publishes its findings in the journal Nature Chemistry.
“In chemistry, cationic low-valent aluminum compounds are highly sought after due to their potential transition metal-like ambiphilic reactivity. However, numerous previous attempts to synthesize cationic low-valent aluminum compounds by oxidative or reductive methods have been largely unsuccessful,” Krossing explains. So far, he said, there has been only one example of a cationic, low-valent aluminum compound, but it cannot be prepared by rational synthesis.
“We now show that there is an unexpectedly easy access to low-valent aluminum complexes with metathesis after all,” Krossing says. In metathesis, partial structures are simply exchanged between the reaction partners.
Aluminum as a cheaper alternative for catalysis
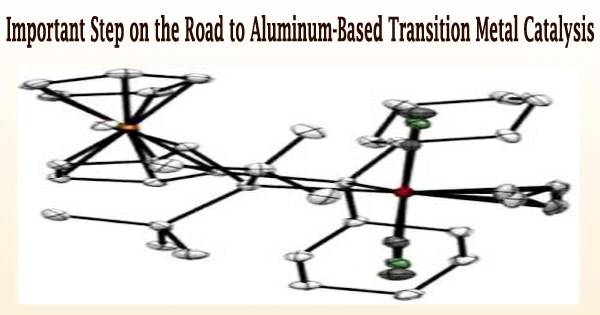
The Schnöckel tetramer (AlCp*)4 was converted by the Freiburg scientists into the salt [Al(AlCp*)3]+[Al(OC{CF3)3}4]-, which already contains aluminum in the +1 oxidation state.
In chemistry, cationic low-valent aluminum compounds are highly sought after due to their potential transition metal-like ambiphilic reactivity. However, numerous previous attempts to synthesize cationic low-valent aluminum compounds by oxidative or reductive methods have been largely unsuccessful.
Profesor Dr. Ingo Krossing
Li[Al{OC(CF3)3}4] and (AlCp*)4 interacted, and the reaction mixture instantly changed from yellow to red. Scientists discovered the [Al(AlCp*)3]+[Al(OC{CF3)3}4] salt as dark purple crystals when the chemical mixture crystallized.
“X-ray crystallographic, UV spectrometric and computational studies indicate the presence of the dimeric structure both in the solid state and in solution at high concentration and low temperature, but at low concentration and room temperature the monomer forms. This clearly indicates ambiphilic reactivity of the cation,” Dabringhaus said.
“Consequently, this salt can potentially be used as a building block for an [:Al(L)3]+-salt that, due to its cationic nature, might be able to perform reversible oxidative additions and reductive eliminations of small molecules,” Krossing explains.
“This brings us one step closer to our long-term goal of achieving catalysis currently done with expensive and rare transition metals with aluminum. Aluminum is the second most abundant element in the Earth’s crust and capable of doing so in principle, as our work shows. But unfortunately, it will probably be at least another 20 years before our research on this is applied.”