Nanoparticles range in size from 1 to 100 nanometers, and unlike regular particles, they are known to have novel highlights that are progressively utilized for diagnosing disease, growing little electronic gadgets, and sunlight-based batteries, as well as in numerous different circles.
In their new paper distributed in Actual Survey B, scientists from Skoltech uncovered that synergist properties of bimetallic nanoparticles—when a material speeds up or postpones a compound response without being consumed by the response—can be calibrated while changing the design of the nanoparticle.
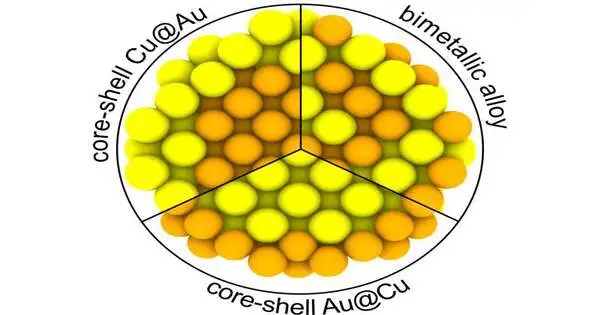
At this point, of most noteworthy interest are bimetallic center-shell particles, in which the center and the shell comprise various metals. Analysts concentrated on three sorts of nanoparticles: Cu-center/Au-shell, Au-center/Cu-shell, and homogeneous bimetallic AuCu combination particles. Dissimilar to center shell particles, the design of regular bimetallic particles isn’t requested.
“We noted that surface electronic states can be altered by varying core-shell ratios. The ability of a nanoparticle to connect with a CO molecule is affected by these modifications. We came to the conclusion that by fine-tuning the core-shell ratio in the nanoparticle, it is feasible to double the adsorption energy—or more accurately, chemisorption, which is a chemical interaction between atoms, molecules of gases, and the surface of the crystal or nanoparticle—in relation to a pure metal.”
Research Scientist Ilya Chepkasov from the Material Discovery Laboratory, the leading author of the study.
“We saw how different center shell proportions can change electronic states on a superficial level. These progressions affect the ability to limit the distance between a nanoparticle and a particle of CO. We presumed that it is feasible to twofold the adsorption energy—all the more exactly, chemisorption, which is a substance restricting between iotas, particles of gases, and the outer layer of the gem or nanoparticle—comparable to an unadulterated metal through tweaking the center shell proportion in the nanoparticle,” said Exploration Researcher Ilya Chepkasov from the Material Disclosure Lab, the main writer of the review.
The review included a few phases and utilized thick practical hypotheses. In the primary stage, the group utilized nanoparticles estimated at 2 nanometers to develop center shell particles with various center shell proportions and broke down how the surface charge changed depending on the proportion. A short time later, the specialists determined the adsorption of CO and O particles on the outer layer of nanoparticles and showed how the adsorption properties of nanoparticles can be changed by fluctuating the surface charge related to calibrating their design.
“We uncovered principal designs that will be subsequently used to foster man-made intelligence-driven models for successful expectation of the adsorption and synergist properties of bimetallic nanoparticles while performing high-throughput evaluation for new materials with indicated properties,” added Teacher Alexander Kvashnin from the Energy Progress Center, at the top of the examination.
The outcomes demonstrate that adjusting the construction of nanoparticles assists with tracking down the fundamental reactant properties of nanoparticles, which will assist with controlling the impetus. Functional importance lies in further developing gas filtration—for instance, for cleaning specialized gases from profoundly harmful CO and making them more secure.
More information: Ilya V. Chepkasov et al, Structure-driven tuning of O and CO adsorption on AuCu nanoparticles: A density functional theory study, Physical Review B (2023). DOI: 10.1103/PhysRevB.108.205414