Scientists at Penn State have effectively 3D bioprinted bosom malignant growth cancers and treated them in a cutting-edge study to more readily comprehend the sickness that is one of the main sources of mortality around the world.
The accomplishment establishes the groundwork for the accurate manufacture of growth models. The headway will empower future review and improvement of anti-malignant growth treatments without the utilization of “in vivo” or “in creature” trial and error.
“This will assist us with understanding how human safe cells cooperate with strong growth,” said Ibrahim Ozbolat, teacher of design science and mechanics, biomedical design, and neurosurgery at Penn State and the senior creator of the review. “We’ve fostered a device that fills in as a clinical test stage to somewhere safe and precisely assess exploratory treatments. It is likewise an examination stage for immunologists and scholars to comprehend how the growth develops, how it collaborates with human cells, and how it metastasizes and spreads in the body. “
Ozbolat’s lab has some expertise in 3D printing to make a variety of tissues for use in human wellbeing. Two diary articles about the lab’s work involving 3D bioprinting to help in the investigation of bosom malignant growth were as of late distributed in Cutting Edge Useful Materials and Biofabrication.
“We have created a tool that may be used as a clinical test platform to safely and effectively assess novel medicines. Additionally, it serves as a study platform for biologists and immunologists to comprehend the growth, interactions, and metastasis of the tumor as well as how it spreads throughout the body.”
Ibrahim Ozbolat, professor of engineering science and mechanics, biomedical engineering.
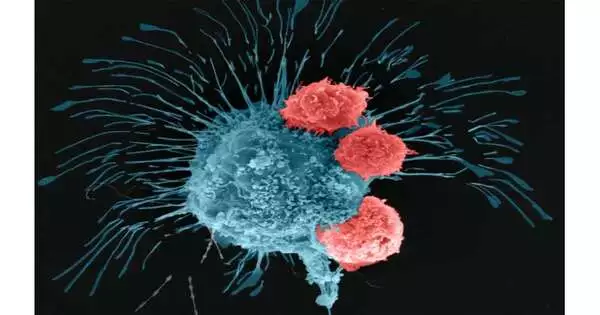
The specialists utilized a new procedure called goal-aided bioprinting to definitively find cancers in three aspects and make the tissue. The specialists then framed the tissue into a multi-scale vascularized bosom cancer model with veins, which they found responded to chemotherapy and cell-based immunotherapeutics.
The group initially verified the exactness of its growth model by treating it with doxorubicin, an anthracycline-based chemotherapeutic medication ordinarily utilized for treating bosom disease. In finding the bioprinted growth responded to chemotherapy, the scientists proceeded to test a cell-put together immunotherapeutic treatment with respect to the cancer in a joint effort with Dr. Derya Unutmaz, an immunologist at Jackson Research Center.
The scientists utilized human vehicle lymphocytes that were designed through quality alteration to perceive and battle a forceful type of bosom disease cell. Following 72 hours of circling the altered vehicle lymphocytes through the growth, the specialists tracked down that the cells inside the bioprinted cancer had created a positive invulnerable reaction and were fending off the disease cells.
“Our model is produced using human cells, but what we make is an extremely worked-on rendition of the human body,” Ozbolat said. “There are many subtleties that exist in the local microenvironment that we can’t reproduce, or even consider duplicating. We are holding back on intricacy. We need to have an essential understanding of how these frameworks work — and we want the development cycle to be smoothed out, in light of the fact that we lack the opportunity to trust that cancers will develop at their regular speed. “
Ozbolat made sense of that, notwithstanding surprising advances in disease therapy, there is an absence of pre-clinical stages for concentrating on trial anticancer specialists. Depending on clinical preliminaries to test the adequacy of therapies eventually restricts the effective clinical interpretation of anti-malignant growth therapeutics, he said. The advancement of bioprinted models could pave the way for totally better approaches for figuring out the growth microenvironment and the body’s safe reaction.
“Immunotherapy has proactively been demonstrated to be a promising treatment for hematologic malignancies,” Ozbolat said. Basically, insusceptible cells of the patient are eliminated and their quality altered to be cytotoxic for disease cells, then, at that point, they are once again introduced into the patient’s circulatory system. Flow is basic on the grounds that the adjusted cells need to move around the body. There is no such thing as with growth, that sort of powerful dissemination, so we constructed our model to attempt to more readily comprehend how cancers respond to immunotherapy.
Growths eliminated from genuine bosom disease patients are presently working with growths. The specialists will apply immunotherapeutics to patient-determined cancers to perceive how they respond.
“This is a significant stage in understanding the complexities of the illness, which is fundamental assuming we will foster novel therapeutics and designated treatments against disease,” Ozbolat said.
More information: Madhuri Dey et al, Chemotherapeutics and CAR‐T Cell‐Based Immunotherapeutics Screening on a 3D Bioprinted Vascularized Breast Tumor Model, Advanced Functional Materials (2022). DOI: 10.1002/adfm.202203966
Madhuri Dey et al, Biofabrication of 3D breast cancer models for dissecting the cytotoxic response of human T cells expressing engineered MAIT cell receptors, Biofabrication (2022). DOI: 10.1088/1758-5090/ac925a
Journal information: Advanced Functional Materials , Biofabrication