Because they are frequently more stable than cells based on liquid electrolytes and have a higher capacity, all-solid-state lithium batteries (ASSLiBs), which are lithium batteries with solid electrolytes, are very promising battery solutions. However, the buildup of lithium inside these batteries can occasionally result in the development of “lithium dendrites,” or “dendritic lithium” (i.e., crystals that resemble trees), which can cause cell short circuits.
Most battery developers currently measure their critical current density (CCD), which is a parameter that determines the maximum current density that the Li/Li symmetric cell can withstand before Li dendrites penetrate through the SSE and cause short circuiting, to evaluate the ability of ASSLiBs to suppress the formation of lithium dendrites. However, in a recent study that was published in Nature Energy, a research team under the direction of Chunsheng Wang, the Robert Franklin and Frances Riggs Wright Distinguished Chair in the Department of Chemical and Biomolecular Engineering (CHBE) and UMD Director of the Center for Research in Extreme Batteries, found another parameter that might be more appropriate for assessing the lithium dendrite suppression abilities of ASSLiBs.
The overpotential, not the CCD, should be what determines the Li-dendrite-suppression capability of solid-state electrolytes (SSEs), according to Hongli Wan, one of the study’s authors, who told Tech Xplore: “We previously found that when decreasing the area-specific resistance of a Li-Li cell, the CCD increased but the overpotential before the voltage drop did not noticeably change. SEI will form at the Li/SSE interface because the majority of SSEs are not stable to Li metal.”.
“We previously discovered that decreasing the area specific resistance of a Li||Li cell increased the CCD but did not significantly change the overpotential before the voltage drop, leading us to believe that it should be the overpotential, not the CCD, that determines the Li-dendrite-suppression capability of solid-state electrolytes (SSEs),”
Hongli Wan, one of the researchers who carried out the study,
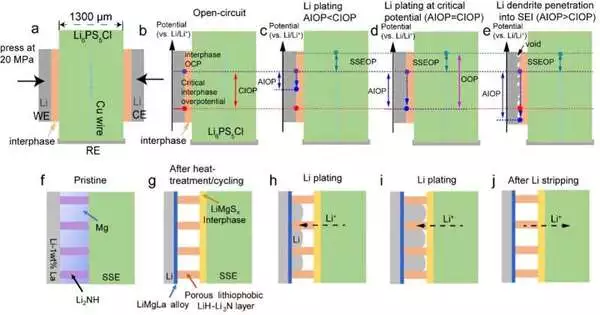
Li diffuses along the Li/SEI interface and maintains a planar growth in ASSLiBs, according to Wang and his coworkers, when Li plating occurs at a low applied interphase overpotential (AIOP). However, Li begins to penetrate the SEI and form the unfavorable dendrites when the AIOP is greater than the critical interphase overpotential (CIOP).
“Li dendrites grow through the SSE, shorting the cell,” explained Wan. “This happens when the applied overpotential is increased further to increase the current density of the CCD.” Li begins to enter the interphase at the CIOP, which is the critical overpotential.
Contrary to the CCD, which varies depending on the SSE integrated in a battery cell’s properties and other engineering factors, the CIOP is a fundamental characteristic of SEIs. This crucial variable is closely related to the lithiophobicity of the electrolyte and interphase (i.e., its mechanical qualities and its innate ability to repel lithium).
This suggests that it might be a more accurate indicator of how well-suited a solid electrolyte is for developing high-capacity ASSLiBs. Additionally, it might serve as a design guide for the interphase inside batteries, enabling researchers to assess how well various interphases suppress lithium dendrites.
According to Wan, the SEI should have a high CIOP (and therefore have a high lithiophobicity and high mechanical strength) to suppress Li-dendrite penetration and a low AIOP (requiring a low SEI resistance and low interfacial resistance) to achieve an all-solid-state lithium-metal battery (ASSLB) with a high energy density. Due to the in-situ formation of Li6PS5Cl/LiMgSx/Li3N-LiH/LiMgLa from Li6PS5Cl/Li2NH-Mg/Li-1.0 wt percentLa after annealing and cycling, our proposed mixed-conductive Li2NH-Mg interlayer can satisfy this requirement.”.
Wang and his coworkers designed an ASSLB with a LiMgSx interphase that they anticipated would aid in preventing the growth of lithium dendrites using CIOP as a guiding parameter. They discovered that the Li plates on the LiMgLa surface reversibly penetrating into porous LiH-Li3N increased the CIOP from about 10 mV (in relation to the SEI of Li6PS5Cl) to about 220 mV, while the AIOP decreased.
This research team’s most recent work demonstrates the value of the CIOP parameter for determining how well solid-state interphases can prevent the growth of lithium dendrites. Future teams could use the newly discovered parameter to design more enticing interphases for ASSLiBs and other solid-state metal batteries, possibly enhancing their performance and capacities.
“We proposed an interphase design criterion (AIOP/CIOP 1) that can make the cell lithium-dendrite-free,” Wan continued. “However, we still haven’t covered all the ways to make sure that the AIOP doesn’t outperform the CIOP. Li diffusivity in the Li anode, void formation at the Li/SSE interface, and the electronic and ionic conductivity of the interphase all have an impact on the AIOP. Our team is methodically analyzing how these elements affect the AIOP.
More information: Hongli Wan et al, Critical interphase overpotential as a lithium dendrite-suppression criterion for all-solid-state lithium battery design, Nature Energy (2023). DOI: 10.1038/s41560-023-01231-w