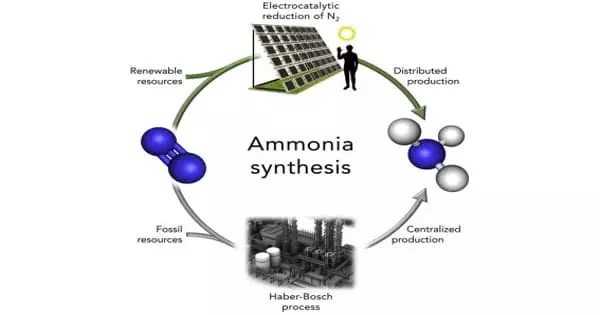
A combined experimental and computational study yields promising results for a new class of ammonia-producing catalysts under mild conditions. Ammonia production is estimated to be around 200 megatons per year. This makes it the world’s second most produced chemical, trailing only sulphuric acid.
There are several methods for producing ammonia, but the Haber-Bosch process is the most common, accounting for roughly 90% of total production. In any case, Haber-Bosch and the other processes used in industrial-scale production necessitate high temperatures (above 400°C) and high pressure (more than 150 bar). These conditions are required for nitrogen’s strong bonds to be broken and for it to react with hydrogen to form ammonia (NH3).
These processes, which account for about 1% of global energy consumption, are primarily fueled by fossil fuels. As a result, ammonia is the most greenhouse gas-intensive chemical-making reaction on the planet, accounting for roughly 1% of total global CO emissions. Furthermore, demand for ammonia is expected to rise in the coming years, owing primarily to its use in synthetic fertilizers required to feed the world’s growing population.
The production of ammonia is one of the major challenges on the climate, energy, and food fronts. It is now manufactured in some of the world’s largest factories. The only truly efficient way to produce ammonia is at high temperatures and pressures with a carbon-based feedstock.
Professor Tejs Vegge
“The production of ammonia is one of the major challenges on the climate, energy, and food fronts. It is now manufactured in some of the world’s largest factories. The only truly efficient way to produce ammonia is at high temperatures and pressures with a carbon-based feedstock “Professor Tejs Vegge of DTU Energy and the VILLUM Center for Sustainable Fuels and Chemicals says (V-Sustain). He co-led the study with Professor Ping Chen of the Chinese Academy of Sciences’ Dalian Institute of Chemical Physics (DICP).
“In enzymes like nitrogenase, nature is very good at producing ammonia at ambient pressures and temperatures. However, the process is extremely slow and cannot be scaled up to industrial production “Tejs Vegge agrees.
Potential game-changer
Scientists have been working hard for decades to develop new and more sustainable methods of producing ammonia. Tejs Vegge and his DTU colleagues, Dr Jaysree Pan and Associate Professor Heine A. Hansen, collaborated with the DICP team to develop a new class of complex metal hydride catalysts that enabled them to achieve the coveted mild-condition ammonia synthesis. They believe their method could pave the way for new, more sustainable ammonia production methods. Their paper was published in the journal Nature Catalysis.
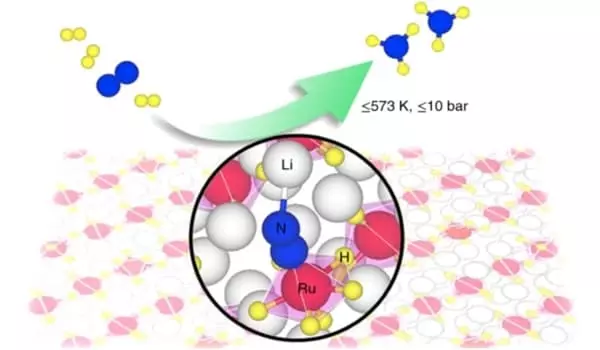
They can synthesize ammonia at temperatures as low as 300°C and pressures as low as 1 bar thanks to their process. The practical application of these catalysts shows promise in terms of small-scale ammonia production using renewable energy. Such systems would typically necessitate catalysts operating at pressures of around 50 bar and temperatures of less than 400°C.
“We believe that our research is unique in that this new class of catalysts exists somewhere between biological and industrial processes. It combines elements from the human, artificial process of heterogeneous catalysis with elements from enzymatic and homogeneous catalysis. It’s an entirely new method of producing ammonia, and we’re combining the best of both worlds, allowing us to significantly lower the temperature and pressure.”
No false notes
In essence, their alternative class of complex metal hydride catalysts (Li4RuH6 and Ba2RuH6) can catalyze the formation of ammonia from hydrogen (H2) and nitrogen (N) (N2). Nitrogen reduction is accomplished through the use of multiple ruthenium hydride complexes, [RuH6]4–, which are rich in electrons and hydrogen. Between the center and the nitrogen, hydrogen transports electrons and protons. Simultaneously, the alkaline metals lithium or barium (Li/Ba) help to stabilize the reaction intermediates. However, the process is highly dynamic, with several parts of the complex serving multiple functions. Calculations alone took years to complete.
“Everything is unlike anything we’ve seen before. Although ruthenium is a well-known component in ammonia catalysis, it exists in a different form and behaves differently. It is surrounded by hydrogen atoms and forms a hydride complex, which allows it to transfer hydrogen in an unusual manner. Imagine this catalyst as a symphony orchestra, where every part must work together to make it work. The most fascinating aspect is that it works, there are no false notes” Tejs Vegge agrees.
“Ammonia catalysis is arguably the most researched catalytic system on the planet. As a scientist, it is extremely satisfying to discover a truly novel mechanism that opens the door to a new world. It may, however, open up new opportunities for ammonia production to take place in a less energy-intensive manner. Today’s large factories are required to make production profitable. Our catalysts or similar ideas could enable production in smaller, decentralized factories. This would also reduce the need for transportation, which currently adds significantly to the cost and CO2 emissions of ammonia.”