A new type of direction-dependent friction in proteins known as anisotropic friction has been discovered by an interdisciplinary research team from the Institutes of Physical Chemistry and Physics of the University of Freiburg and the Max Planck Institute of Biophysics in Frankfurt am Main.
Nobody had previously noticed that the direction of friction in biomolecules is reversed, according to physicist Dr. Steffen Wolf of the University of Freiburg. The findings were published in the journal Nano Letters.
Experiments with a model protein-ligand complex
Cellular micro-engineering is made up of proteins. Throughout their functional cycles, they are working. As a result, they obey the laws of thermodynamics, have a high efficiency of energy conversion, and dissipate energy throughout their functional cycle. The latter effect corresponds to apparent friction from a macroscopic standpoint.
“We assume that this hitherto unknown and essential sort of friction exists in any bioassembly when randomization in protein orientation arises alongside directionality of force application,”
Dr. Bizan N. Balzer, a biophysicist.
Internal protein friction, which is caused by the excitation of protein-internal vibrations, is a well-known source of friction on the microscopic scale of individual proteins. Solvent friction, which results from the acceleration of nearby solvent molecules, is another source. These frictional sources cause the solvent and protein to heat up. By performing single-molecule experiments and simulations on a model complex of a protein and a ligand, the researchers were able to identify the novel type of friction in this instance.
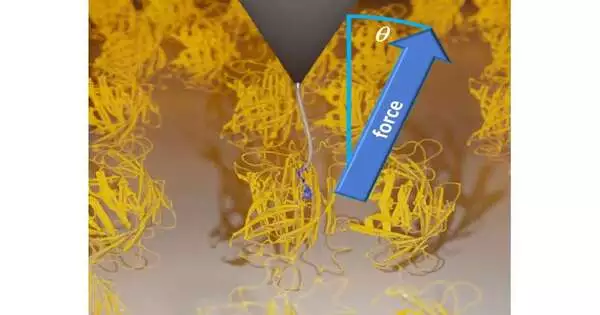
The team used stereographic single-molecule force spectroscopy, a novel technique based on atomic force microscopy (AFM), in their single-molecule experiments. They were able to use this method to examine the dissociation of a ligand from a protein bound to a surface along each of the three Cartesian coordinates.
In the course of their research, Dr. Wanhao Cai, Prof. Dr. Thorsten Hugel, Dr. Bizan N. Balzer, and Dr. Jakob T. Bullerjahn of the Max Planck Institute made the startling discovery that friction during ligand unbinding rises with the applied pulling angle.
Combining computer simulations and experiments.
Later, the experiment was re-created by Miriam Jäger and Dr. Steffen Wolf of the University of Freiburg’s Institute of Physics using computer simulations. They utilized the BinAC-HPC-Cluster in Tübingen’s high-performance computing (HPC) resources. They discovered through the simulations that the precise direction in which the pulling force is applied affects how much work is required to separate a ligand from its binding site.
The proteins bound to the surface in the experiment had an undefinable and random orientation along their rotational axes. By combining the results from the experiment and simulations, the researchers realized that this orientation is what causes the angle-dependent friction. In order to get statistically significant results, the team repeated the single-molecule pulling experiments numerous times by binding and unbinding a ligand from and to a protein.
For each measurement, a different protein is the ligand’s binding site. As a result, a ligand was pulled over various areas of the randomly oriented protein in each measurement, but at the same angle to the surface. This orientation cannot be defined, either in the experimental setup or in the real world, and each measurement cannot be precisely and irreversibly repeated. As a result, the energy deposited in the biomolecule varied each time.
This energy’s irreversible portion was lost to the system as heat. The researchers refer to the resulting effect as anisotropic friction, which is a source of friction.
A basic form of friction.
In every bioassembly where randomness in protein orientation appears along with directionality of force application, according to Dr. Bizan N., “we assume that this previously unknown and fundamental type of friction is present.”. a biophysicist named Balzer. He explains that this is the case for processes like blood flow, where forces are applied to randomly oriented proteins, or for biomolecular motors or force-sensitive membrane proteins.
“Anisotropic friction is therefore another crucial piece of the puzzle for understanding friction in both technical applications and in biological complexes generally,” says Balzer in his conclusion. “.
More information: Wanhao Cai et al, Anisotropic Friction in a Ligand-Protein Complex, Nano Letters (2023). DOI: 10.1021/acs.nanolett.2c04632