Two new strains of bacteria in the microbiome of mice that produce inflammatory bowel disease (IBD) symptoms have been discovered and named by researchers from the Wellcome Sanger Institute, the Hudson Institute of Medical Research in Australia, the University of Cambridge, and partners.
The study, published this month in Nature Microbiology, demonstrates that the bacteria are widely present in mice used to investigate IBD and may have an impact on the outcomes of future research into the condition. This demonstrates the importance of considering the gut microbiome composition when evaluating results. In the future, examining whether similar strains can be detected in the human gut could lead to a greater understanding and treatment of IBD.
Inflammatory bowel disease, or IBD, is a chronic disorder that affects around 6.8 million individuals worldwide each year. It is caused by chronic gastrointestinal inflammation and encompasses ulcerative colitis and Crohn’s disease. IBD is a chronic disorder that can cause debilitating symptoms that significantly affect a patient’s quality of life when it flares up.
While the precise origin of IBD is unknown, it has been proposed that in certain people, the immune system reacts to naturally occurring bacteria in the gut, emphasizing the importance of investigating the microbiome in understanding and treating this condition.
Although our understanding of the human microbiome has grown significantly in recent years, comparable studies in mouse models have remained limited. To understand what is happening in the gut and the role of genetics in this illness, researchers mainly rely on a mouse model known as the dextran sodium sulfate, or DSS, model.
While specialized techniques have been devised to prevent any effects produced by recognized bacterial pathogens in the mouse microbiota, there is still a diverse variety of bacteria that need to be classified, identified, and understood. Understanding the mouse microbiota and its impact on disease is critical in case this changes outcomes and has implications for future research.
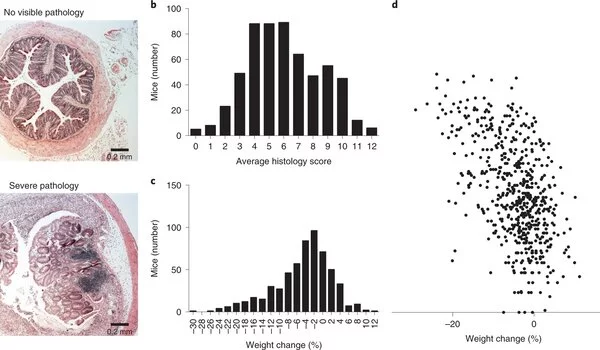
In this recent article, researchers tested 600 mice from a single site at the Wellcome Sanger Institute and thoroughly examined each microbiome. Duncaniella muricolitica and Alistipes okayasuensis were named after the researchers who discovered two new bacteria that were causing weight loss and intestinal inflammation in mice.
Using data from a prior study that cataloged 26,640 mouse microbiome bacteria, they discovered that D. muricolitica and A. okayasuensis are widespread bacteria in mouse colonies all around the world.
“Mouse models are used widely to uncover more information on the development and treatment of inflammatory bowel disease, however our research shows that the microbiome of these mice could impact on the results from these studies. While there are currently methods in place to reduce the impact of known disease-causing bacteria on studies, we have highlighted that there are bacteria that were previously unknown, and therefore not adjusted for. We hope that our research helps in the study design and interpretation of similar work in the future,”
Dr. Samuel Forster, first author from the Hudson Institute of Medical Research, Australia.
Because these bacteria generate IBD symptoms, they have an effect on the outcomes of IBD mouse models. Researchers recommend that this be recognized when designing experiments, and that if these bacteria are present, the interpretation of the results should take this into account.
“Mouse models are commonly utilized to learn more about the development and treatment of inflammatory bowel disease, but our findings suggest that the microbiome of these mice may have an impact on the outcomes of these studies. While techniques are currently in place to decrease the impact of known disease-causing bacteria on research, we have highlighted that there are bacteria that were previously unknown and hence not accounted for. We hope that our research will be useful in the design and interpretation of similar studies in the future. First author Dr. Samuel Forster, first author from Australia’s Hudson Institute of Medical Research, states
“The composition of the human gut microbiome is becoming more recognized as having an important role in health and disease, particularly in disorders such as inflammatory bowel disease. To research this, we also need a better understanding of the mouse microbiome, including which bacteria are present, how they interact, and how these vary across mouse colonies and facilities. Our research is beginning to provide fresh and important insights into these issues, “Dr. Virginia Pedicord, senior author from the University of Cambridge, agrees.
“Our large-scale study discovered two new strains of bacteria that induce illness symptoms in mice. We now have a better understanding of the results of future investigations utilizing these mouse models because we are aware of these germs. This is significant because scientists conducting basic research as well as biotechnological or pharmaceutical investigations into inflammatory bowel disease around the world use mouse models to discover new ways to prevent or treat this condition. Trevor Lawley is a senior author at the Wellcome Sanger Institute.





