A scalable hydrothermal method for obtaining ferromagnetic single-atom spin catalysts that can improve the efficiency of water splitting reactions induced by a magnetic field has been developed by chemists at the National University of Singapore (NUS).
Electrolysis of water utilizing inexhaustible assets is a promising innovation for hydrogen creation and is gaining interest around the world. This is because hydrogen is regarded as a promising alternative to address the growing concerns regarding the potential climate change-causing carbon emissions from fossil fuels.
However, the oxygen evolution reaction’s (OER) sluggish kinetics are the primary constraint on hydrogen production through water electrolysis. Valuable metal-based electrocatalysts are frequently utilized in the OER cycle to work on their unfortunate effectiveness. When combined with an applied magnetic field, a novel class of catalytic materials known as heterogeneous ferromagnetic single-atom spin catalysts (SASCs) has a great potential to accelerate this chemical reaction.
“This discovery shows that ferromagnetic SASCs have a strong magnetoelectric effect that speeds up the electrolysis of water. The method presents unheard-of possibilities for the creation of ferromagnetic SASCs based on non-precious metals for both water and saline water electrolyzer technologies.”
Professor Xin Luo from Sun Yat-sen University, China,
Be that as it may, the plan for such productive and strong SASCs for the OER cycle is not yet perceived as a significant test.
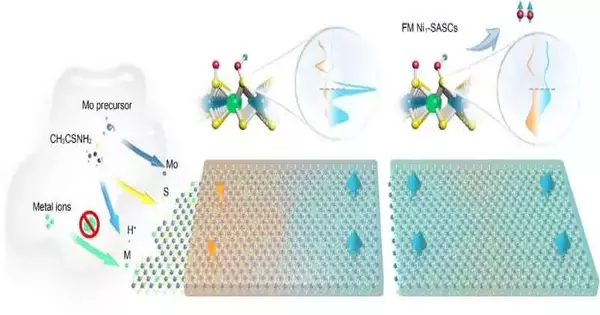
A general and scalable hydrothermal method for the synthesis of a series of SASCs with tunable high loading was developed by the NUS Department of Chemistry research team led by Associate Professor Lu Jiong. Metal salts (M2+), ammonium molybdate tetrahydrate, and thioacetamide can all be introduced during a single processing step as a result of this.
The significant development lies in the formation of operando acidic circumstances during union that can stifle the conglomeration into metal nanoparticles by the dopant. The dopant can achieve high-density substitution, which is necessary for the spin catalyst’s proper formation and the ferromagnetic effect, by dispersing itself uniformly as single atoms on the material.
Professor Xin Luo from Sun Yat-sen University in China, Dr. Shibo Xi from the Institute of Sustainability for Chemicals, Energy, and Environment in Singapore, and Professor Cheng-Hao Chuang from Tamkang University in Taiwan are all partners in this endeavor.
This examination advancement was distributed in the journal Nature Nanotechnology.
The host material was molybdenum disulfide (MoS2), and the researchers discovered that the various M1/MoS2 SASCs (M1=Mn, Fe, Ni, and Co) can have a doping ratio of up to 20:100. By and large, higher doping thickness lessens the typical dividing line between neighboring attractive dopant destinations and expands the long-range attractive request.
Under commonly available magnetic fields, this study demonstrates the conceptual design and synthesis of a new class of robust ferromagnetic SASCs with giant magnetic field enhancement (MFE). These SASCs can be used to accelerate the electrolysis of water and saline water.
The design of advanced heterogeneous spin catalysts with atomically precise active sites to boost reaction kinetics and probe their mechanistic insights remains challenging, despite the fact that the MFE effect on chemical reactions has been exploited in homogenous spin catalysis systems as well as in bulk solid catalysts (such as mixed oxides and metals).
This is due to the need to deal with multiple complexities, such as the ability to engineer distinct active sites with strong local magnetism (local magnetism) and global magnetism (long-range ferromagnetic ordering) and short-range quantum spin exchange interaction (QSEI) within each site.
Beyond the synthesis aspect, this work’s main novelty goes beyond that. All of the SASCs developed by the researchers exhibit interatomic QSEI to induce local magnetic moments, with spin density delocalized over adjacent sulfur atoms via strong p-d orbital hybridization, according to joint experimental and theoretical studies.
In their tests, including the utilization of the Ni1/MoS2 SASC, a gentle attractive field of around 0.5 tesla (regularly open attractive fields from super durable magnets) when applied yields a sensational improvement of OER magnetocurrent by around 3,000%. When compared to commercial iridium dioxide (IrO2) catalysts, it also demonstrated excellent reactivity and stability in both pure water splitting cells and cells that split water from seawater.
Assoc. Prof. Lu said, “This finding uncovers that ferromagnetic SASCs give a strong magnetoelectric impact to speed up water electrolysis. For the design of non-precious metal-based ferromagnetic SASCs for both water and saline water electrolyzer technologies, this method provides unprecedented opportunities.”
“We intend to chip away at the huge scope union of ferromagnetic single-iota turn impetuses joined with attractive fields to foster arrangements that can decrease the expense and work on the effectiveness of green hydrogen creation from the electrolytic parting of water and seawater,” added Assoc. Prof. Lu.
More information: Tao Sun et al, Ferromagnetic single-atom spin catalyst for boosting water splitting, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01407-1