Manganese oxides stand out from materials researchers because of their inescapable applications, including anodes, impetuses, sensors, supercapacitors, and biomedicine. Further, manganese is broadly bountiful and has numerous oxidation states, which permits it to shape different fascinating translucent designs.
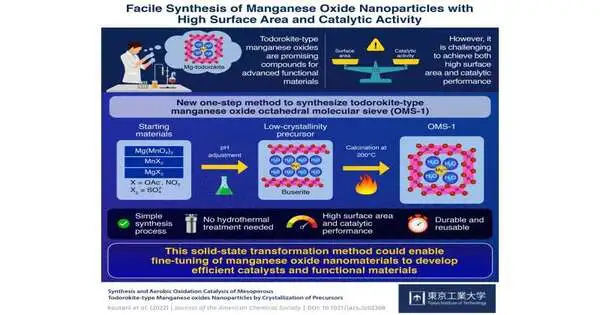
One such design is the “todorokite-type manganese oxide octahedral sub-atomic sifter (OMS-1),” a gem whose unit cells (easiest rehashing units of the precious stone) are comprised of three-by-three MnO6 octahedral chains. However, as an impetus, the capability of OMS-1 is restricted for two reasons. To begin with, its regular amalgamation strategies are perplexing multi-step crystallization processes, including aqueous or reflux treatment. Second, these cycles will quite often make precious stones with a higher molecule size and a lower surface region, highlighting impediments to synergist execution.
In order to bypass these issues, an examination group from the Tokyo Institute of Technology (Tokyo Tech) thought of a basic method for blending OMS-1 nanoparticles. Driven by Associate Professor Keigo Kamata, the group found that the way to effectively deliver excellent OMS-1 was to utilize forerunners with low crystallinity. Their review was published in the Journal of the American Chemical Society. Also, the logical representation of this review, made by Dr. Kamata, was chosen as a supplementary cover art for the diary.
“Our catalyst had a specific surface area of about 250 m2/g, which is significantly more than the 185 m2/g that the OMS-1 synthesized using previously reported methods could reach.”
Dr. Kamata
They considered their clever union system the “strong state change technique.” In it, one must first join explicit proportions of MnO4- and Mn2+ reagents, such as Mg(MnO4)2 and MnSO4.In the wake of changing the pH of the combination, one requirement is to gather the accelerated once they settle. These are predominantly comprised of low-crystallinity Mg-buserite, a sort of layered manganese oxide. The buserite is then calcinated at 200 °C for 24 hours, which changes it into OMS-1 nanoparticles.
Through different analyses performed utilizing advanced hardware, the group completely portrayed the OMS-1 they created. They decided the ideal boundaries to acquire the best return on the response and the best quality OMS-1. The momentous part of the pre-arranged OMS-1 nanoparticles was their surface region, as featured by Dr. Kamata: “Our impetus displayed a particular surface area of around 250 m2/g, which is a lot bigger than that of OMS-1 combined utilizing recently detailed techniques, which just went up to 185 m2/g.”
To scrutinize the incorporated OMS-1, the specialists researched its synergist execution for different liquor oxidation responses with oxygen (O2) as the main oxidant. The outcomes were exceptionally uplifting. Dr. Kamata remarks: “The OMS-1 orchestrated through our methodology is a powerful and reusable heterogeneous impetus for the oxidation of different kinds of sweet-smelling alcohols and sulfides.” Regardless of our nanoparticles’ being minuscule, they displayed no compromise between surface region, molecule size, and reactant execution. “
Generally speaking, the discoveries of this study shed light on the most efficient method to more readily control the amalgamation of manganese oxide nanoparticles. These experiences will ideally lead not exclusively to profoundly productive impetuses but, in addition, to novel manganese oxide-based useful materials with pragmatic applications.
More information: Maki Koutani et al, Synthesis and Aerobic Oxidation Catalysis of Mesoporous Todorokite-Type Manganese Oxide Nanoparticles by Crystallization of Precursors, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c02308
Journal information: Journal of the American Chemical Society