Infection-prompted respiratory diseases can become perilous. A group at the Paul-Ehrlich-Institut, along with scientists in Heidelberg, has found that seasonal infection contamination restricted to the lungs likewise prompts the enactment of blood immature microorganisms and the expanded development of platelets. Platelets can cause apoplexy, as has been demonstrated in extreme instances of coronavirus. The messenger substances interleukin-1 and interleukin-6 are associated with the initiation. Cell Reports accounts for the outcomes in its web-based release from October 4, 2022.
Influenza flare-ups of varying power happen consistently in the cold weather months. They are brought about by flu infections. Serious instances of flu contamination are related to the wrecking of the insusceptible framework, a cytokine storm with the extreme arrival of courier substances (cytokines), and harm to lung cells. Contamination can prompt vein breaks and cause apoplexy. These responses are like extreme instances of the coronavirus brought about by the SARS-CoV-2 covid. When do serious cases arise and what cycles happen when this occurs? Many subtleties are obscure.
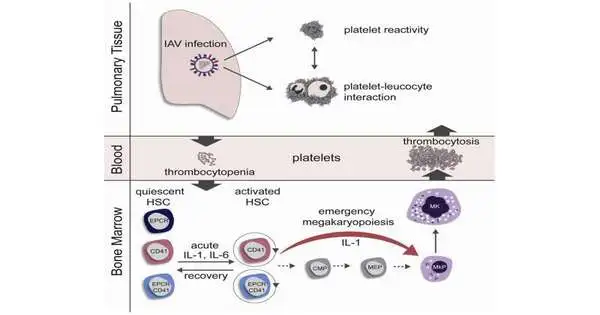
Difficulties of severe influenza cases include both a decreased and increased number of platelets in the blood, which can be linked to an increased risk of apoplexy.The specialists, led by Ute Modlich, head of the exploration group “Quality Change in Foundational Microorganisms” at the Paul-Ehrlich-Institut, have worked in an examination network with researchers at the German Disease Exploration Center (DKFZ) Heidelberg and the College of Heidelberg on the connection between a seasonal infection contamination (H1N1) and the development of blood or platelets. H1N1 flu infections are among the flu infections that circulate every year in Germany.
Disease of the lungs with flu infections and its impact on blood arrangement
All platelets are recharged in this manner by foundational microorganisms that can form into platelets (hematopoietic undeveloped cells, HSCs). These cells exist in a resting state in the bone marrow. The destiny of the HSCs is completely controlled between rest, self-recharging, and separation to guarantee long-lasting hematopoiesis.
To research the impact of flu disease on blood arrangement, mice were tainted intranasally with flu infections and their blood foundational microorganisms were examined in this manner concerning separation and cell cycle actuation. In the initial three days of intense flu disease, the platelets at first diminished (thrombocytopenia), but at that point rose quickly in the blood to levels above physiological levels (thrombocytosis). These quickly created platelets had a youthful appearance (aggregate) and were all the more quickly activated (hyper-receptive).
An expanded number of blood undifferentiated organisms were found in the development cycle only two days after disease (G1 and S/G2/M cell cycle stages). The enactment of the blood foundational microorganisms was related emphatically with the viral lung titer, i.e., the more infections had gone after the lungs, the more blood undifferentiated cells were actuated. Contaminations with decreased flu portions postponed immature microorganism actuation, but couldn’t forestall it. In the recovery stage, the blood foundational microorganisms get back to the resting stage. This happened quicker in immunized mice than in different gatherings.
Blood foundational microorganisms with average markers for platelet antecedents
To explain how platelets can be delivered so rapidly, the examination group investigated the aggregate of the initiated blood undifferentiated organisms and found that a subset of the blood immature microorganisms previously conveyed regular markers of platelet forerunner cells (megakaryocytes). Blood undeveloped cells with this surface aggregate separate straightforwardly into megakaryocytes and platelets. They avoid a few stages by going before.The examination bunch showed through in vitro heredity following and bone marrow transfers that this gathering of blood immature microorganisms quickly duplicates in the bone marrow after flu disease. These newly created platelets are bigger and more juvenile in appearance than common platelets and will generally enact all the more quickly, which can prompt a higher risk of blood clusters in the lungs.
The process of rapid separation of megakaryocytes has undoubtedly previously been depicted as crisis megakaryopoiesis, which happens as a result of fundamental irritations or contaminations.Up until this point, in any case, no relationship with nearby popular respiratory illnesses has been thought of. Although the flu infection disease in mice was confined to the respiratory tract, expanded levels of the cytokines interleukin-1 (IL-1) and interleukin-6 (IL-6) were tracked down in the bone marrow of contaminated mice. Using two groups of knockout mice, one with the IL-1 receptor turned off and one with the IL-6 receptor turned off, the examination group showed that these cytokines contribute definitively to the enactment of the blood undeveloped cells and to crisis megakaryopoiesis in flu contaminations.
The ongoing research shows how even a nearby (non-foundational) viral contamination can cause changes in blood development in the bone marrow. Platelets shaped in this cycle can prompt a higher risk of blood clumps, specifically in the lungs, in the hyper-responsive state. This might affect genuine influenza cases.
More information: Marcel G.E. Rommel et al, Influenza A virus infection instructs hematopoiesis to megakaryocyte-lineage output, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111447
Journal information: Cell Reports