People aren’t the main focus for infections. Microbes, like humans, become contaminated by a variety of infections.In reality, for billions of years, microbes and infections have been engaged in a never-ending development weapons competition for endurance that includes endless advancements and counter-variations.
As of late, biomedical researchers have shown an increasing interest in infections known as bacteriophages, or phages, which can taint and kill risky microbes. Phages, the most bountiful creatures on earth, are presently perceived as a promising device for fighting bacterial disease as science looks for new treatments for rising rushes of anti-toxin resistance. Researchers might want to explore the mysteries of phages’ developmental systems in their continuous clash with microbes.
A gathering of scientists with different fortes across the University of California San Diego grounds brings currently utilized new innovations to the table for experiences into previously unnoticed phage organic designs and cycles. Distributed in the journal Nature, they offer an uncommon opportunity to investigate an under-concentrated family known as “large phages” and their amazingly advanced guards against microbes.
“This compartment is unusual from anything we have ever seen in nature. We were able to characterize this compartment—how it assembles and functions at the most fundamental level—from each atom to the scale of the entire organism.
Elizabeth Villa, an associate professor in the UC San Diego School of Biological Sciences
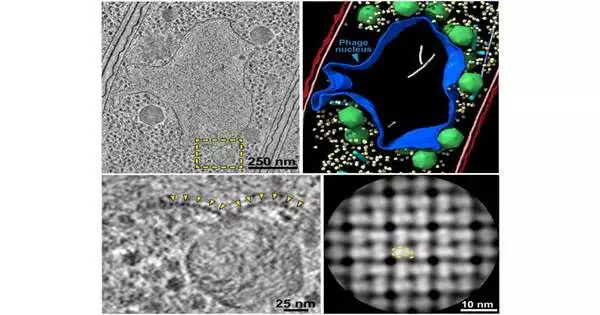
Among their revelations, researchers from the labs of Elizabeth Villa, Kevin Corbett, and Joe Pogliano found that large phage cells build a safeguarded compartment that acts like a core in human and creature cells and safeguards the infection’s center hereditary material, which is expected to repeat and spread. The exploration group described the design of the core like compartment interestingly utilizing driving advances, including cryo-electron microscopy and tomography, at the most elevated goal feasible for cell imaging.
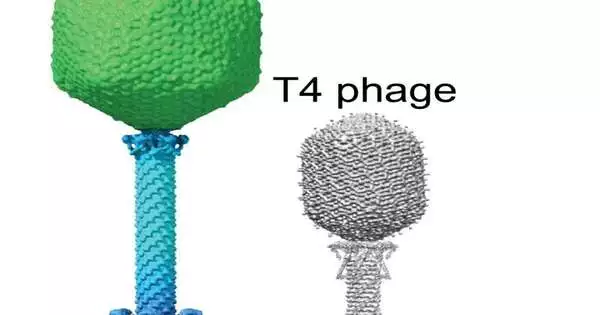
A cryo-EM portrayal of a large phage contrasted with a T4 phage infection and a ribosome molecule. Villa Lab at UC San Diego
“It’s an alternate sort of compartment—dissimilar to anything we have at any point found in nature,” said Villa, an academic partner in the UC San Diego School of Biological Sciences and a Howard Hughes Medical Institute Investigator. “We had the option to portray this compartment — how it gathers and works at the most essential level — from every iota to the size of the whole creature.”
Branch of Chemistry and Biochemistry Professor Rommie Amaro and her partners then applied cutting-edge computational methods to mimic the phage design’s capabilities and amazing adaptability. The scientists found that the compartment permits specific key parts inside while at the same time filling in as a guard system against bacterial dangers.
“These disclosures present us with a totally different time of phage science,” said Villa. “The shell fills in as a developing safeguard for security, yet it likewise needs to import and produce a few things, and it does this with choice accuracy and selectivity.” It’s very odd science. “
The scientists found that the phage’s core-like shell is gathered from a solitary protein. Given its job in phage guard, they named the protein chimallin after the safeguard conveyed by old Aztec heroes.
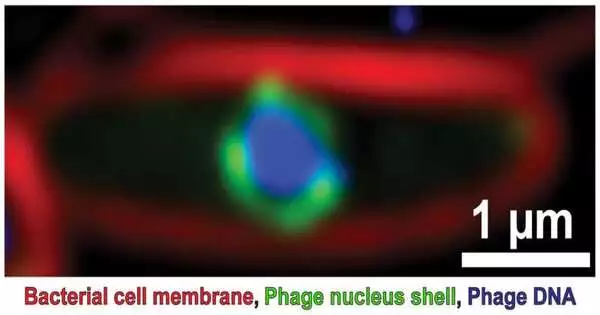
A fluorescence microscopy picture uncovers a bacterial cell tainted by a large phage, uncovering the bacterial cell film (red), the phage core shell (green) and phage DNA (blue). Pogliano Lab, University of California, San Diego
Concentrate on co-creator Joe Pogliano, a teacher in the Department of Molecular Biology, who has been reading up on these phages for over 10 years. He accepts core framing phages could be better for phage treatments against bacterial diseases since they have evolved to be normally impervious to many kinds of bacterial guard frameworks.
“As we push toward the improvement of phage treatments, we’ll have to study this newfound phage core since it seems to improve their ability to go after microbes,” said Pogliano. Analysts, including Pogliano and Villa, will team up with specialists in UC San Diego’s Center for Innovative Phage Applications and Therapeutics, the main devoted phage treatment focus in North America. “Now that we realize specific phages have a safeguard, we could give it to different phages and make’super phages’ that are better at phage treatment and beating bacterial guards.” The most vital phase in that cycle is understanding the design of the chimallin protein that makes up the safeguard, which is one explanation this work is so significant. “
Teacher Kevin Corbett, an individual from the Department of Cellular and Molecular Medicine, added natural chemistry and primary science skills to the examination group. He depicts the discoveries to act as an illustration of united advancement in which remotely related creatures track down comparable ways of tackling issues.
“The atomic pore in eukaryotes is a huge, complex design with particular approaches to keeping most proteins out yet explicitly bringing in others.” “What we’re likely taking a gander at with the large phage is a decisively easier strategy for tackling a similar issue,” said Corbett. “It’s an incredibly savvy fix—comparable yet easier—to shielding its genome from the rest of the world by building a wall to isolate it from bacterial guards.”
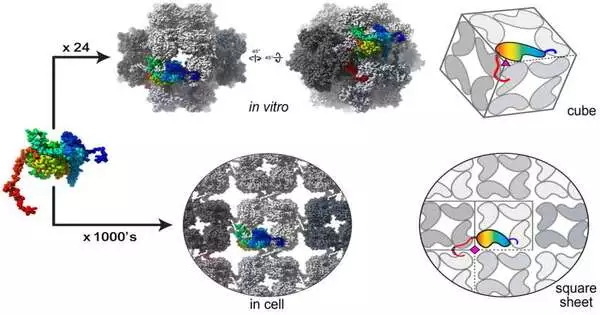
24 individual chimallin proteins gather as a shape inside the cells of tainted microbes. The great many chimallin parts gather into tiles that at last make up square sheets of the phage core shell. Villa Lab at UC San Diego
Laughlin, a Biological Sciences postdoctoral researcher, drove the perception of the large phage compartment. Using offices and mechanical assets novel to UC San Diego, and in a joint effort with co-first creator Amar Deep and different individuals from teaming up labs, they described the compartment from the size of microns to iotas to assist with unraveling its capabilities.
Laughlin said he was generally shocked at observing that the compartment is shaped with various duplicates of the chimallin protein organized as a square grid, or fishnet-like setup. Since honeycomb (hexagonal) setups are considerably more typical in nature, Laughlin and different individuals from the group didn’t expect such a basic design to hide the compartment’s construction.
Laughlin and different analysts say their discoveries regarding the large phage and its compartment lead to a lot more inquiries, including how certain parts are handled inside and outside the shell.
Laughlin said, “We presently realize the rule design of the compartment of a developed phage core, yet we might want to know how it gathers regardless.” “What is the biogenesis (or “prequel”) at the beginning phases of disease?” How can everything begin once the infection infuses its genome into the host microbes? “
The Nature paper’s full creator list incorporates Thomas Laughlin, Amar Deep, Amy Prichard (graduate understudy), Christian Seitz (graduate understudy), Yajie Gu, Eray Enustun (graduate understudy), Sergey Suslov, Kanika Khanna (late Ph.D. beneficiary), Erica Birkholz (graduate understudy), Emily Armbruster (graduate understudy), J. Andrew McCammon, Rommie Amaro, Joe Pogliano, Kevin Corbett, and Elizabeth Villa.
More information: Thomas G. Laughlin et al, Architecture and self-assembly of the jumbo bacteriophage nuclear shell, Nature (2022). DOI: 10.1038/s41586-022-05013-4
Journal information: Nature