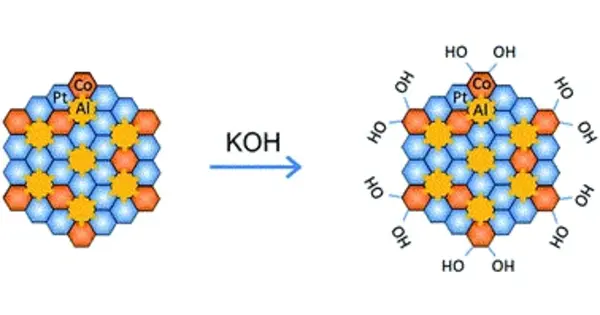
Hydrogen has the highest energy density (120 MJ/kg) of all known substances, approximately three times more than diesel or gasoline, meaning it could play a pivotal role in sustainable energy systems. But the efficient production of hydrogen by simple water splitting requires highly performing catalysts. Now, a collaborative group from Tohoku University and Johns Hopkins University have developed nanoporous molybdenum-based intermetallic compounds that could boost hydrogen production.
Intermetallic compounds in nano-scale formed from non-precious transition metals have the potential to be cost-effective and robust catalysts for hydrogen production. However, the development of monolithic intermetallic compounds, with ample active sites and sufficient electrocatalytic activity, remains a challenge for scientists.
Our research has played a crucial part in addressing that problem. Focusing on design and engineering, we harnessed an advanced dealloying technique for constructing the intermetallic compounds’ architecture.
Professor Hidemi Kato
“Our research has played a crucial part in addressing that problem,” says Professor Hidemi Kato, from the Institute for Materials Research at Tohoku University and co-author of the study. “Focusing on design and engineering, we harnessed an advanced dealloying technique for constructing the intermetallic compounds’ architecture.”
Liquid metal dealloying is a processing technique that utilizes the difference in alloy components’ miscibility in a molten metal bath to corrode selected component(s), while retaining the others. It allows for self-organizing into a three-dimensional porous structure.
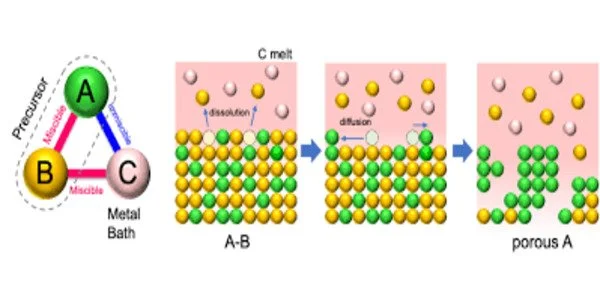
Furthermore, it enables the pore size to be controlled at the nanometer scale for both μ-Co7Mo6 and μ-Fe7Mo6, which are generally at the micrometer scale for the other metals/alloys when coarsening takes place at equivalent temperatures.
The collaborative group then researched the electrocatalytic performance of the new nanoporous intermetallic compounds. It showed promise and potential for use as a commercial HER catalyst for high-current applications.
The results of their research were published in the journal Nature Communications. Looking ahead, the research group hopes to use liquid metal dealloying to develop more monolithic nanoporous intermetallic compounds by exploring the fundamental mechanisms behind general intermetallic phases.