Of the numerous ways of treating disease, the most seasoned, and perhaps generally proven, is a medical procedure. Indeed, even with the coming of chemotherapy, radiation treatment, and more trial therapies like microbes that look for and obliterate disease cells, tumors simply should be removed from a patient’s body.
The objective is to eliminate all of the harmful tissue while saving as much of the encompassing sound material as could be expected. But since it tends to be hard to define a perfect boundary between harmful and solid tissues, specialists frequently decide in favor of wariness and eliminate sound tissue to ensure they have taken out the entirety of the dangerous tissue.
This is particularly risky when a patient is experiencing a disease that besets bones; bones present novel difficulties during medical procedures due to how hard they are compared to different tissues and on the grounds that they recover considerably more leisurely than different sorts of tissue.
“It’s difficult to develop bone, so assuming you cut out bone, you essentially lose it,” says Bren Teacher of Clinical Designing and Electrical Designing Lihong Wang.
Another analytic imaging innovation developed by Caltech analysts allows specialists to make cuts multiple times more precisely, allowing them to save up to multiple times more sound tissue and give patients easier recuperations.
The customary techniques used to decide if a piece of bone contains disease cells are tedious. The lump of bone is taken out and shipped off to a lab where its hard calcium grid is gradually broken up, abandoning just the residing cells. The excess material is then cut and imaged. Since the cycle can require anywhere somewhere in the range of one to seven days to finish, specialists can’t depend on it during a medical procedure to decide the strength of the bone around and close to a growth. Thus, they will eliminate a much greater amount of it than may be needed — and more than they would in milder tissues that can be rapidly biopsied.
The new imaging innovation, called 3D form-check bright photoacoustic microscopy, or UV-PAM, is intended to supplant the customary strategy for recognizing harmful bone tissue. Since the cycle requires only minutes, it gives a specialist the capacity to separate solid bone from harmful bone while they work.
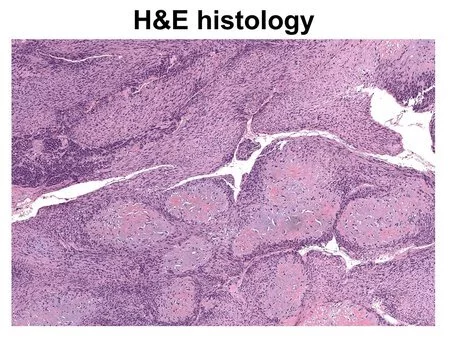
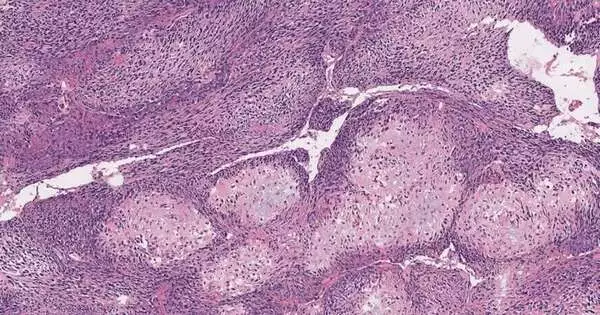
A picture of harmful tissue is ready with the customary hematoxylin and eosin (H&E) staining strategy.
Like other photoacoustic imaging advances created by Wang, UV-PAM works by utilizing laser light to make atoms in living tissue vibrate. Those vibrations occur at ultrasonic frequencies and can be utilized to picture tissues and organs in a similar way that ultrasound is utilized to picture a developing baby.
UV-PAM utilizes bright frequencies of laser light tuned to make atoms of DNA and RNA vibrate. Since disease cells are organized in an unexpected way, stuffed more thickly, and contain considerably more DNA than solid cells, an area of harmful tissue will retain a greater amount of the UV light and hence will give a more grounded ultrasonic sign than sound tissue, making it feasible for the specialist to plainly recognize areas of bone that should be taken out.
“We can give results in the span of 11 minutes, so they know precisely where to cut,” Wang says.
The innovation furnishes specialists with a picture of the bone that they have checked that is arranged to seem to be the pictures that are made through customary biopsy methods.
“We simply present pictures to the pathologists,” Wang says. They utilize a similar example of acknowledgment in their own mind to figure out what is harmful and what is solid. That is their preparation. “
At this moment, the innovation is just being shown in a lab setting. Wang says he wants to bring it into the present day, where it is used on patients, but first he wants to make some improvements.
“We might want to give it a much better spatial goal and more prominent imaging speed so we might see a portion of the subtleties inside the cell core more quickly,” he says. “Might we at any point go past standard pathology? We are dealing with that. “
The paper depicting UV-PAM shows up in Nature Biomedical Design.
More information: Rui Cao et al, Label-free intraoperative histology of bone tissue via deep-learning-assisted ultraviolet photoacoustic microscopy, Nature Biomedical Engineering (2022). DOI: 10.1038/s41551-022-00940-z
Journal information: Nature Biomedical Engineering