Science is increasingly utilizing the tricks plants can perform with photosynthesis: driving compound reactions that run inefficiently or do not happen suddenly with light energy.This requires reasonable photocatalysts that catch light energy and make it accessible for the response. A Chinese research team has now published layered center-shell quantum dabs that effectively drive testing natural changes in the journal Angewandte Chemie.Their low level of harmfulness is a specific benefit.
Quantum dabs are finely scattered nanoscopic gems of inorganic semiconductors. They stay firmly in a movable range of the range and are easy to reuse.Only recently have photocatalytic quantum dots been assembled with regard to the extremely hazardous components cadmium and lead.This and their restricted proficiency have been the primary hindrances to their more extensive use.
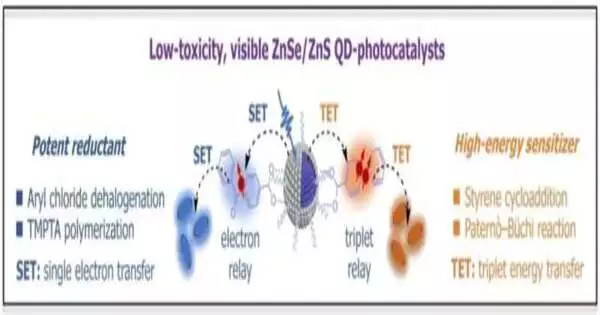
An exploration group led by Kaifeng Wu (Chinese Institute of Sciences) has now presented novel quantum dots with low harmfulness and elite execution. They are enacted by monetarily accessible blue LEDs; the UV light that is normally required isn’t required. The key to their prosperity lies in their center/shell structure and the variable coatings that can be utilized to “store” the light energy.
The quantum dots are a couple of nanometers wide. Their center consists of zinc selenide (ZnSe) and is encircled by a slim shell made of zinc sulfide (ZnS). Blue light raises the zinc selenide to an energized state, where it can undoubtedly surrender electrons. The shell keeps the electrons from being quickly caught by alleged deserts.
The group prepared the outer layer of the shell with unique benzophenone ligands that “kiss up” the electrons from the quantum dabs, store them, and make them accessible for natural responses. For instance, the group had the option to do reductive dehalogenations of aryl chlorides and add substance-free polymerizations of acrylateshesignificant responses that are run inadequately or not at all by regular photocatalysts.
A subsequent form was made by covering the surface with biphenyl ligands that can straightforwardly ingest energy from energized quantum dots. This transforms them into an enduring, profoundly fiery trio.The trio energy “put away” in this way can be moved to explicit natural particles, which then likewise enter a trio state. In this state, they can go through compound reactions that are unrealistic in their ground state.
As a demonstration, the group performed [2+2] homo-cycloadditions of styrene and carbonyl cycloadditions with alkenes. These produce four-membered rings (cyclobutanes or oxetanes, separately), which are substances that are significant starting materials in regions like drug improvement.
More information: Chengming Nie et al, Low‐Toxicity ZnSe/ZnS Quantum Dots as Potent Photoreductants and Triplet Sensitizers for Organic Transformations, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202213065
Journal information: Angewandte Chemie International Edition , Angewandte Chemie