As they develop, strong cancers encircle themselves with a thick, difficult-to-enter mass of sub-atomic guards. Getting drugs past that blockade is notoriously troublesome. Presently, researchers at UT Southwestern have created nanoparticles that can separate the actual boundaries around growth to arrive at disease cells. When inside, the nanoparticles discharge their payload: a quality-altering framework that changes DNA inside the cancer, impeding its development and enacting the safe framework.
The new nanoparticles, described in Nature Nanotechnology, really halted the development and spread of ovarian and liver tumors in mice. The framework offers another way ahead for the utilization of the quality altering device known as CRISPR-Cas9 in disease treatment, said concentrate on pioneer Daniel Siegwart, Ph.D., Associate Professor of Biochemistry at UT Southwestern.
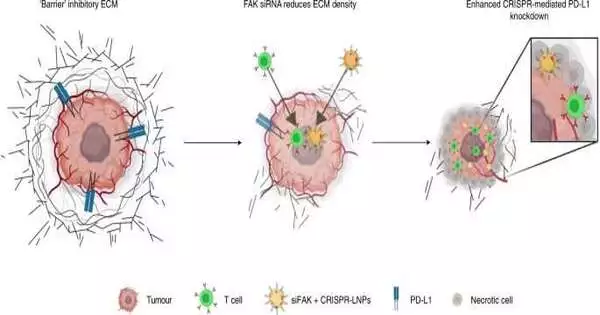
“Targeting FAK not only reduces the barrier around tumors and makes it easier for nanoparticles to enter the tumor, but it also opens the door for immune cells to enter,”
Di Zhang, Ph.D
“Despite the fact that CRISPR offers another methodology for treating disease, the innovation has been seriously upset by the low proficiency of conveying payloads into growth,” said Dr. Siegwart, an individual from the Harold C. Simmons Comprehensive Cancer Center.
Recently, the CRISPR-Cas9 innovation has provided scientists with a method to specifically alter the DNA inside living cells.While the quality altering framework offers the possibility to modify qualities that are driving disease development, conveying CRISPR-Cas9 to strong cancers has been tested.
For over 10 years, Dr. Siegwart and his partners have been examining and planning lipid nanoparticles (LNPs), little circles of greasy atoms that can convey sub-atomic freight (counting late mRNA COVID-19 antibodies) into the human body. In 2020, Dr. Siegwart’s group determined the best way to coordinate nanoparticles to explicit tissues, which had been a test restricting the field.
In the new work, to target disease, the analysts started with the nanoparticles that they had previously advanced to go to the liver. They added a little piece of RNA (called short meddling RNA or siRNA) that could shut down central bond kinase (FAK), a quality that plays a central part in keeping intact the actual guards of various cancers.
“Focusing on FAK not only weakens the blockade around cancers and makes it easier for the nanoparticles themselves to advance into the growth, but it also prepares to allow safe cells in,” said Di Zhang, Ph.D., a postdoctoral research individual at UTSW and the paper’s first author.
Inside the recently designed nanoparticles, the analysts typified CRISPR-Cas9 hardware that could alter the quality of PD-L1. Numerous diseases utilize this quality to create elevated levels of the PD-L1 protein, which slows down the safe framework’s capacity to go after growth. Researchers have recently demonstrated the way that upsetting the PD-L1 quality in certain tumors can lift those brakes and empower an individual’s safe framework to kill disease cells.
Drs. Siegwart, Zhang, and their partners tried the new nanoparticles in four mouse models of ovarian and liver disease. They initially showed that by adding siRNA to stop FAK, the grid of atoms around the growth was less firm and simpler to enter than typical. Then, they examined the cancer cells and found that a lot more nanoparticles had arrived at the phones, really changing the PD-L1 quality.
Finally, they found that tumors in mice treated with the nanoparticles that were designated both FAK and PD-L1 shrank to around one-eighth the size of cancers treated exclusively with void nanoparticles. Overall, about two times as lengthy.
More work is expected to show the security and viability of the nanoparticles in an assortment of growth types. The analysts said the treatment might be helpful in relation to existing disease immunotherapies that plan to utilize the safe framework to go after growth.
“After the overall outcome of the COVID-19 LNP antibodies, we are considering what else LNPs can do.” Here we grew new LNPs fit for conveying various sorts of hereditary medications all the while working on helpful results in disease. “There is plainly incredible potential for LNP meds to treat various types of illnesses,” said Dr. Siegwart.
More information: Di Zhang et al, Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy, Nature Nanotechnology (2022). DOI: 10.1038/s41565-022-01122-3