Scientists at the Tokyo Foundation for Innovation and the Public Organization for Combination Science have explained the compound similarity between high-temperature fluid metal tin (Sn) and decreased enactment ferritic martensitic, a competitor’s primary material for combination reactors.
This revelation has prepared for the improvement of a fluid metal tin divertor, which is a high-level intensity expulsion part of combination reactors. To keep up with the benefits of plasma, a device known as a divertor is used in combination reactors.For diverters, there has been interest in fluid metals that can endure huge intensity loads from high-temperature plasma.
Combination reactors are effectively evolving all over the world as a type of feasible zero-carbon energy on the grounds that their fuel can be removed from an endless stock of seawater. Likewise, they don’t radiate ozone-harming substances. Notwithstanding the development of the tokamak (ITER), which is a cooperation of seven of the world’s leading nations and locales (Japan, the EU, the US, South Korea, China, Russia, and India), combination improvement in the confidential area is likewise speeding up.
“Although liquid metal tin is a good coolant with a wide range of qualities, it corrodes structural materials. We think that by explaining the corrosion mechanism, we can encourage the use of liquid metal tin for solar thermal power plants as well as fusion energy.”
Associate Professor Masatoshi Kondo of Tokyo Institute of Technology,
Perhaps the main part of these combination reactors is the divertor, a part that gasifies pollutants in the plasma and sends the gas to a fume siphon. During the operation of a combination reactor, a portion of the divertor’s primary parts are subjected to very high intensity loads on a par with “space transport while entering the air.”Scientists are attempting to foster a strong divertor in which a block of intensity-safe material, for example, tungsten, is put in touch with the plasma and cooled with high-temperature, high-pressure water.
This strong divertor framework is likewise utilized in the ITER project and the model combination reactors. On the other hand, as a creative system to endure the huge intensity load from plasma, scientists have likewise viewed the idea of a fluid metal divertor, which shields the divertor from plasma by covering the primary material of the divertor with a fluid metal that has great cooling performance.
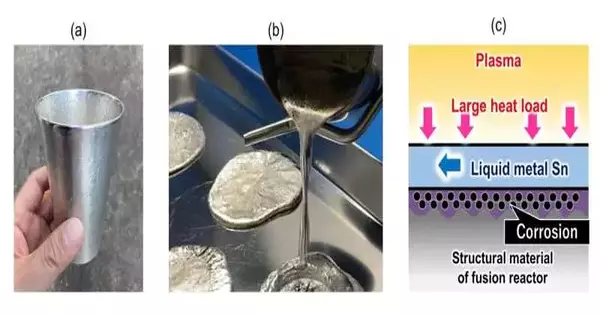
As displayed in Fig. 1(a), tin (Sn) is a metal that has been utilized in different ways in our regular routines; for instance, as a material for flatware and as a part of welding. Tin has a somewhat low softening mark of 232 °C and is reasonable for use in a fluid state, as displayed in Fig. 1 (b). One more property of tin is that its fume strain at high temperatures is lower than that of other fluid metals.
Hence, as displayed in Fig. 1(c), when fluid metal tin is utilized as a coolant to cover and safeguard the primary material surface of the fluid metal divertor of a combination reactor, it is impervious to vanishing regardless of whether it is warmed by plasma and arrives at a high temperature. Likewise, any vanished metal is more averse to blending in with the plasma. In any case, the erosion of primary materials is a specialized issue that has been of concern to the scientists.
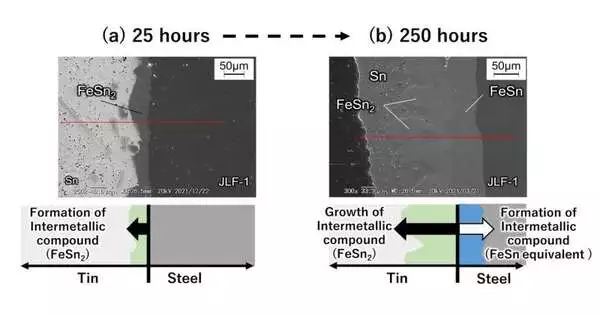
Figure 2: A cross-section of the surface layer of the combination reactor primary material (lower enactment ferritic martensitic steel) drenched in fluid tin.(a) When drenched in fluid tin at 500 °C for 25 hours. (b) When drenched in fluid tin at 500 °C for 250 hours taken utilizing a checking electron magnifying lens.
Kondo’s lab has centered around the compound conjunction of different primary and useful materials. The lab has given specific consideration to fluid metal coolants that stand out in the field of cutting-edge energy, like combination reactors. Analysts concentrated on the fluid metal tin, which has the peculiar property of being extremely receptive at high temperatures. They attempted to explain the erosion system of primary materials used in combination reactors and to identify materials that exhibit consumption opposition.
What is high-temperature fluid metal tin, and what causes its serious destructiveness?
Decreased actuation The ferritic-martensitic (Fe-9Cr-2W-0.1C) primary material for combination reactors is dependent on a piece of ferritic-martensitic heat-safe steel.Reduced enactment ferritic-martensitic uses additional substance components that reduce induced radioactivity, anticipating use in a neutron-light environment in a reactor.
When low-enactment ferritic martensitic steel comes into contact with fluid metal tin, the hatching time frame before erosion begins is brief.As displayed in Figures 2(a) to 2(b), the analysts verified that the steel contains iron (Fe), which responds with high-temperature tin to erode the material while quickly shaping intermetallic compounds (FeSn2, and so on) on the tin.
As well as containing iron, decreased-enactment ferritic martensitic steel likewise contains components, for example, chromium and tungsten, that don’t readily react with tin. Hence, the steel has a lower erosion rate than that of unadulterated iron. In any case, following ten days at 500 °C, the steel frames an intermetallic compound with a thickness of around 155 micrometers and erodes. While extrapolating these numbers to one year, the thickness could reach the requested millimeters, which is a huge erosion rate. At 600 °C, the analysts discovered that the deterioration caused by erosion was much more severe.As of now, the analysts likewise found that erosion advances because of the internal dispersion of tin into the microstructure of the steel.
Academic partner Masatoshi Kondo of the Tokyo Foundation for Innovation, who drives the exploration group, gave the accompanying clarification: “Albeit fluid metal tin is a great coolant with various properties, it has the downside of eroding primary materials.” “By explaining the erosion system, we desire to advance the utilization of fluid metal tin for combination energy as well as for sun-based nuclear energy stations.”
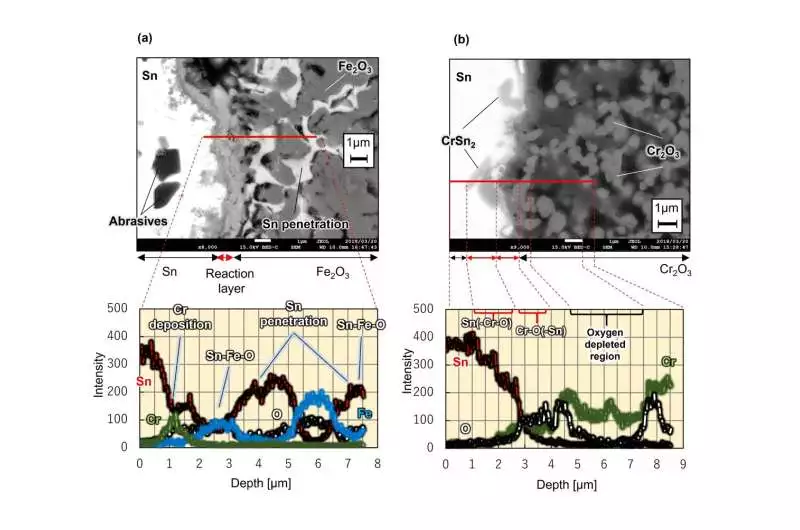
Figure 3: A cross-section of an oxide sinter surface layer erosion design drenched in fluid metal tin.(a) When a sintered group of iron oxide (Fe2O3) was drenched in fluid tin at 500 °C for 262 hours. (b) When a sintered group of chromium oxide (Cr2O3) was drenched in fluid tin at 500 °C for 262 hours. Photographs were taken with a checking electron magnifying lens.
What materials can withstand the high-temperature fluid metal tin?
The analysts tracked down the fact that steel and iron-based primary materials erode, both obviously and internally, while shaping intermetallic compounds in high-temperature fluid metal tin. This is on the grounds that iron, the primary part of steel, reacts with high-temperature fluid tin. Hence, the scientists guessed that it would be feasible to forestall the response with high-temperature tin by joining the iron with oxygen ahead of time to form an oxide that preceded the response.
Chasing after this hypothesis, the analysts tried iron oxide (Fe2O3) and chromium oxide (Cr2O3) for similarity with fluid tin at 500 °C. The outcomes are displayed in Figure 3. Tin entered the pores formed during terminating while drenching the iron oxide sintered material.Nonetheless, the thickness of the response structure with tin on the material surface was around 1 micrometer. This was a very slim response, which is just around 1% of the decrease in actuation in ferritic steel.
Furthermore, when analyzing the sintered material of chromium oxide, it is observed that the response structure with tin is slim on a superficial level.Along these lines, the scientists found that even a metal component, for example, iron, which effectively responds with tin, can be essentially stifled by an earlier response with oxygen to frame an oxide.
“The working climate of a fluid tin divertor in a combination reactor is under very cruel circumstances in which erosion by fluid tin and light from combination neutrons are superimposed,” made sense of Teacher Kondo, who leads research by the Japanese group in Errand 3 of the U.S.-Japan Science and Innovation Collaboration Program: Outskirts Task.
“In this task, we are collaborating with venture colleagues from Oak Edge Public Lab in the United States to investigate the effects of radiation on the erosion response elements of steel with fluid tin,” he explained.
The disclosures examined in this article explained the cause and system of fluid metal tin erosion, which has areas of strength to some extent. This exploration will contribute essentially to the accomplishment of a carbon-unbiased society by aiding the improvement of profoundly solid, high-level intensity gear for combination reactors.
More information: Masatoshi Kondo et al, Corrosion mechanism of reduced activation ferritic martensitic steel JLF-1 in liquid metal Sn, Corrosion Science (2022). DOI: 10.1016/j.corsci.2022.110748