Battery-powered lithium-particle batteries power telephones, workstations, other individual hardware, and electric vehicles, and are even used to store energy created by sunlight-based chargers. Yet, assuming the temperature of these batteries ascends excessively high, they quit working and can burst into flames.
That is to a limited extent in light of the fact that the electrolyte within them, which ships lithium particles between the two terminals as the battery charges and releases, is combustible.
“Quite possibly the greatest test in the battery business is this wellbeing issue, so there’s a ton of exertion going into attempting to make a battery electrolyte that is protected,” said Rachel Z Huang, an alumni undergrad at Stanford College and the first creator of a report distributed in Issue.
“This new discovery suggests a new way of thinking about polymer-based electrolyte design. This electrolyte is critical for designing future batteries with great energy density while being safe.”
Zhenan Bao, a professor at Stanford University and investigator
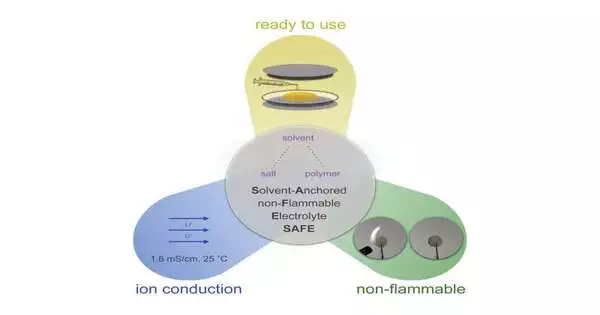
Huang fostered a non-combustible electrolyte for lithium-particle batteries with 19 different specialists at the Division of Energy’s SLAC Public Gas Pedal Lab and Stanford College. Their work exhibited that batteries containing this electrolyte keep working at high temperatures without lighting a fire.
Their mystery? More salt.
Pungent Wellbeing
Customary lithium-particle battery electrolytes are made of a lithium salt that breaks down in a fluid that is naturally dissolvable, like ether or carbonate. While this dissolvable further develops battery execution by assisting with moving lithium particles around, it’s likewise a potential firestarter.
Batteries produce heat as they work. Furthermore, if a battery experiences penetrations or surrenders, it will quickly warm up.At temperatures over 140 degrees F, the little particles of dissolvable in the electrolyte begin to dissipate, changing from fluid to gas and blowing up a battery like an inflatable—until the gas bursts into flames and the situation disintegrates.
Throughout the course of recent years, specialists have created non-combustible electrolytes, for example, polymer electrolytes, which utilize a polymer network rather than the exemplary salt-dissolvable solution to move particles around. In any case, these more secure options don’t move particles as proficiently as fluid solvents do, so their performance hasn’t compared to that of traditional electrolytes.
The group needed to deliver a polymer-based electrolyte that could offer both well-being and execution. What’s more, Huang had a thought.
She chose to add however much she could of a lithium salt called LiFSI to a polymer-based electrolyte planned and orchestrated by Jian-Cheng Lai, a postdoctoral researcher at Stanford College and co-first author on the paper.
“I simply needed to perceive the amount I could add and test the breaking point,” Huang said. Normally, less than half of a polymer-based electrolyte’s weight is salt. Huang knocked that number to 63%, making one of the saltiest polymer-based electrolytes of all time.
Unlike other polymer-based electrolytes, this one contained combustible dissolvable particles.Notwithstanding, the general electrolyte, known as dissolvable Moored non-combustible electrolyte (SAFE), demonstrated non-combustibility at high temperatures during tests in a lithium-particle battery.
SAFE works on the grounds that the solvents and salt work together. The dissolvable atoms assist with leading particles, bringing about execution similar to that of batteries containing customary electrolytes. Be that as it may, rather than coming up short at high temperatures like most lithium-particle batteries, batteries containing SAFE keep on working at temperatures between 77 and 212 degrees F.
In the interim, the more than adequate added salts act as anchors for the dissolvable atoms, keeping them from dissipating and bursting into flames.
“This new finding brings up a better approach for thinking about polymer-based electrolyte configurations,” said Zhenan Bao, a teacher at Stanford College and specialist with the Stanford Organization for Materials and Energy Sciences (SIMES), who exhorts Huang. “This electrolyte is important for developing future batteries with high energy density while remaining safe.”
Remaining gooey
Polymer-based electrolytes can be strong or fluid. Critically, the solvents and salt in SAFE plasticize its polymer network to make it a goo-like fluid, very much like regular electrolytes.
One advantage: a gooey electrolyte can squeeze into existing, financially accessible lithium-particle battery parts, not at all like other non-combustible electrolytes that have arisen. Strong state earthenware electrolytes, for example, should use exceptionally planned cathodes, making them prohibitively expensive to deliver.
“With Protected, there’s no compelling reason to change any of the assembly arrangements,” Huang said. “Obviously, if it is ever used for production, improvements are required for the electrolyte to enter the production line, but the work is significantly different from any of the other frameworks.”
Yi Cui, a teacher at SLAC and Stanford and a SIMES examiner who likewise exhorts Huang, said, “This exceptionally intriguing new battery electrolyte is viable with the current lithium particle battery cell innovation and would have large effects on shopper gadgets and electrical transportation.”
One utilization of SAFE might be in electric vehicles.
Assuming that the numerous lithium-particle batteries in an electric vehicle sit excessively near one another, they can warm each other up, which could ultimately prompt overheating and fire. Yet, assuming an electric vehicle contains batteries loaded up with an electrolyte like SAFE that is steady at high temperatures, its batteries can be stuffed near one another without stress of overheating.
As well as reducing fire risk, this implies less space involved in cooling frameworks and more space for batteries. More batteries increase the general energy thickness, meaning the vehicle could go longer between charging.
“So it’s not only a wellbeing benefit,” said Huang. “This electrolyte could likewise permit you to pack in much more batteries.”
More information: Zhuojun Huang et al, A solvent-anchored non-flammable electrolyte, Matter (2022). DOI: 10.1016/j.matt.2022.11.003
Journal information: Matter