Almost all traction batteries currently used in EVs and consumer electronics require lithium. Lithium-ion (Li-ion) batteries are also widely used in a variety of other applications, including energy storage and air mobility. Because battery content varies depending on the active materials mix, and new battery technologies are entering the market, there are many unknowns about how the battery market will affect future lithium demand.
A lithium metal anode, for example, which increases energy density in batteries, has nearly double the lithium requirements per kilowatt-hour when compared to the current widely used mixes that include a graphite anode.
The irregular movement of lithium ions in next-generation battery materials has been discovered by researchers to be reducing capacity and hindering performance. The team, led by the University of Cambridge, used real-time tracking to monitor the movement of lithium ions inside a promising new battery material.
The mechanism by which lithium ions are stored in battery materials was assumed to be uniform across individual active particles. The Cambridge-led team discovered, however, that lithium storage is anything but uniform during the charge-discharge cycle.
Our model provides insights into the range over which lithium-ion diffusion in NMC varies during the early stages of charging. Our model predicted lithium distributions accurately and captured the degree of heterogeneity observed in experiments. These predictions are key to understanding other battery degradation mechanisms such as particle fracture.
Dr Shrinidhi S. Pandurangi
When the battery is nearing the end of its discharge cycle, the active particles’ surfaces become lithium saturated while their cores are lithium deficient. As a result, reusable lithium is lost, and capacity is reduced.
The Faraday Institution-funded research could help improve existing battery materials and speed up the development of next-generation batteries. The findings were published in Joule.
The use of electric vehicles (EVs) is critical in the transition to a zero-carbon economy. Because of their high energy density, lithium-ion batteries power the majority of electric vehicles on the road today. However, as EV use becomes more widespread, the push for longer ranges and faster charging times means that current battery materials need to be improved, and new materials need to be identified.
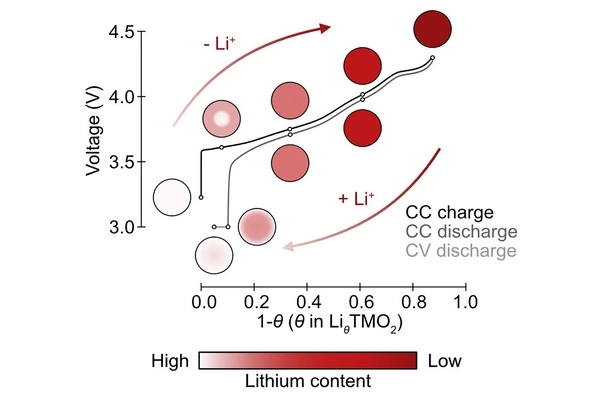
Some of the most promising of these materials are layered lithium nickel-rich oxides, which are widely used in premium EVs and are state-of-the-art positive electrode materials. However, their working mechanisms, particularly lithium-ion transport under practical operating conditions and how this relates to their electrochemical performance, are not fully understood, so we are unable to achieve maximum performance from these materials.
The researchers observed distinct differences in lithium storage during the charge-discharge cycle in nickel-rich manganese cobalt oxide batteries by tracking how light interacts with active particles during battery operation under a microscope (NMC).
“This is the first time that non-uniformity in lithium storage has been directly observed in individual particles,” said Alice Merryweather, co-first author from Cambridge’s Yusuf Hamied Department of Chemistry. “We need real-time techniques like ours to capture this while the battery is cycling.”
The researchers discovered that the non-uniformity is caused by drastic changes in the rate of lithium-ion diffusion in NMC during the charge-discharge cycle by combining experimental observations with computer modeling. Lithium ions diffuse slowly in fully lithiated NMC particles, but diffusion is significantly increased once some lithium ions are extracted from these particles.
“Our model provides insights into the range over which lithium-ion diffusion in NMC varies during the early stages of charging,” said co-first author Dr Shrinidhi S. Pandurangi from Cambridge’s Department of Engineering. “Our model predicted lithium distributions accurately and captured the degree of heterogeneity observed in experiments. These predictions are key to understanding other battery degradation mechanisms such as particle fracture.”
Importantly, the lithium heterogeneity seen at the end of discharge explains why nickel-rich cathode materials typically lose around 10% of their capacity after the first charge-discharge cycle.
“This is significant because one industrial standard used to determine whether a battery should be retired or not is when it has lost 20% of its capacity,” said co-first author Dr Chao Xu of ShanghaiTech University. The researchers are now looking for new ways to improve the practical energy density and lifetime of these promising battery materials.