Lutein is a xanthophyll compound that is bountiful in egg yolks, natural products, and vegetables. It protects the eye from oxidative damage caused by radiation and reduces the risk of eye diseases such as macular degeneration and cataracts.Merchandised items including lutein are gotten from the concentrates of the marigold bloom, which is known to hold onto bountiful measures of lutein. Nonetheless, the downside of lutein creation from nature is that it requires a long time to develop and reap marigold blossoms. Besides, it requires extra physical and compound-based extractions with a low yield, which makes it monetarily impossible. The significant expense and low yield of these bioprocesses have made it hard to fulfill the need for lutein promptly.
These difficulties roused the metabolic designers at KAIST, including analysts Dr. Seon Young Park, Ph.D. applicant Hyunmin Eun, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering. The group’s review was published in Nature Catalysis on August 5, 2022.
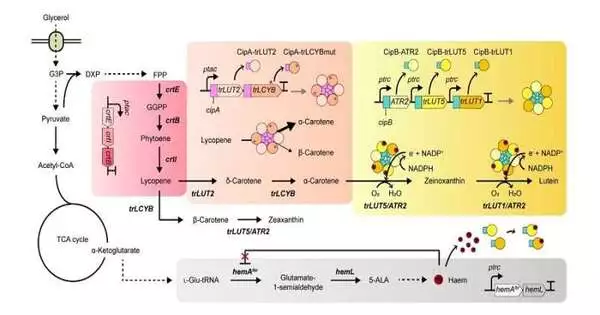
This examination subtleties the capacity to create lutein from E. coli with a high return by utilizing a modest carbon source, glycerol, through framework metabolic design. The examination bunch zeroed in on tackling the bottlenecks of the biosynthetic pathway for lutein creation, built inside a singular cell. To begin with, utilizing frameworks for metabolic designing, which is a coordinated innovation to design the digestion of a microorganism, lutein was created when the lutein biosynthesis pathway was presented, but in tiny sums.
“As the necessity of sustaining good health in an aging society grows, we anticipate that the technology and tactics established here will play critical roles in creating other useful natural products of medical or nutritional importance.”
Professor Sang Yup Lee
To work on the efficiency of lutein creation, the bottleneck proteins inside the metabolic pathway were first recognized. It turned out that metabolic responses that include a wanton protein, a chemical that is engaged with at least two metabolic responses, and electron-requiring cytochrome P450 compounds are the primary bottleneck steps of the pathway hindering lutein biosynthesis.
To overcome these challenges, substrate directing, a method of falsely selecting proteins in the cell’s actual vicinity to create nearby groups of substrates that can be converted into items, was used to channel more metabolic motion towards the target compound while reducing the development of undesirable results.
Furthermore, electron diverting, a system similar to substrate directing but differing in that it expands the nearby groupings of electrons required for oxidoreduction responses interceded by P450 and its reductase accomplices, was used to smooth out the metabolic motion towards lutein biosynthesis, resulting in the most elevated titer of lutein creation accomplished in a bacterial host ever revealed. A similar electron diverting system was effectively applied for the creation of other normal items incorporating nootkatone and apigenin in E. coli, exhibiting the overall relevance of the system in the examination field.
“It is normal that this microbial cell industrial facility-based creation of lutein will actually want to supplant the ongoing plant extraction-based process,” said Dr. Seon Young Park, the main creator of the paper. She reasoned that one more significant finding of the study is that the coordinated metabolic designing systems developed from this research can be broadly applicable to the effective creation of other common items such as drugs or nutraceuticals.
“As keeping up with great wellbeing in a maturing society is turning out to be progressively significant, we expect that the innovation and systems created here will assume vital parts in delivering other important normal results of clinical or dietary significance,” made sense of by Distinguished Professor Sang Yup Lee.
More information: Sang Lee, Metabolic engineering of Escherichia coli with electron channelling for the production of natural products, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00820-4. www.nature.com/articles/s41929-022-00820-4