An Empa researcher named Qun Ren and her team are currently working on a diagnostic method that can quickly identify staphylococcal blood poisoning, which can be fatal.
Up to 40% of cases of staph infection lead to death. The spherical bacterial infection may have begun as a local skin disease or pneumonia. In the course of sepsis, the staphylococci can swarm into the bloodstream, causing severe complications. In situations like these, the pathogens need to be found as soon as possible, and the right antibiotics need to be chosen for treatment.
Because Staphylococcus aureus strains can be insensitive to a variety of antibiotics, this is especially important for the chances that the affected people will be able to survive. “Valuable time is lost if a blood sample’s bacteria must first be cultivated for a diagnostic procedure,” says Qun Ren, the group leader at Empa’s Biointerfaces lab in St. Gallen. As a result, Qun Ren and Fei Pan, a teammate, collaborated with researchers from ETH Zurich to find a way to skip the lengthy intermediate step.
“As a next step, we’d like to validate the sepsis tests in collaboration with our clinical partners by analyzing patient samples,”
Qun Ren, an Empa researcher,
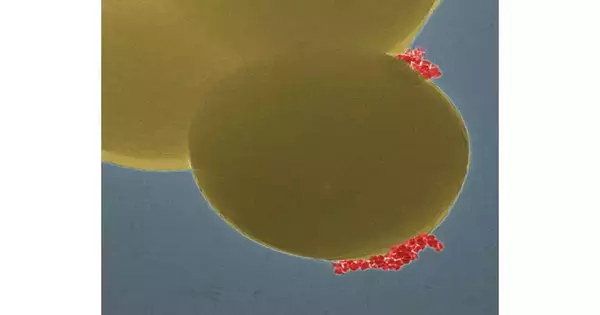
The group has fostered a strategy utilizing attractive nanoparticles that can bind to staphylococci. As a result, a magnetic field can specifically detect the bacteria. In the following stage, the aversion to anti-infection agents is examined using a chemiluminescence strategy. The sample gives off light if the bacteria in the test tube are resistant. On the other hand, the reaction vessel stays dark if antibiotics can kill the germs. All things considered, the sepsis test requires something like three hours, contrasted with a few days for the exemplary development of bacterial societies,” says Fei Dish.
Pseudomonas aeruginosa is another unpleasant member of the bacterial kingdom. During a hospital stay, this rod-shaped bacterium can cause a variety of diseases, including infections of the urinary tract. Sepsis is a condition that can result from these infections. Additionally, these pathogens frequently possess antibiotic resistance.
This is where the magnetic nanoparticles’ additional benefit comes into play: Similar to a modular system, the method can be tailored to many different kinds of bacteria. In this manner, a magnetic nanoparticle-based rapid “sepsis sensor” was developed by Empa researchers. Using a chemiluminescence reaction, the method reliably identified the bacterial species in samples containing artificial urine and identified potential antibiotic resistance.
Laboratory samples have been used so far by the researchers to test their magnetic nanoparticle kit for sepsis and urinary tract infections. In a subsequent stage, we might want to approve the sepsis tests along with our clinical accomplices by assessing patient examples,” says Qun Ren.
The examination is distributed in the journals ACS Sensors, Biosensors, and Bioelectronics.
Around the world, the declining viability of anti-microbials causes more than 1,000,000 infections every year. Some staphylococci, for instance, have developed resistance, so common antibiotics no longer work to control them. Particularly concerning is the proportion of pathogens that are multiresistant. Pathogen antibiotic resistance is already being referred to as a “silent pandemic” worldwide. The speed and accuracy with which a germ is identified when diagnosing an infection can be crucial to the infected person’s survival.
More information: Fei Pan et al, Ultrafast Determination of Antimicrobial Resistant Staphylococcus aureus Specifically Captured by Functionalized Magnetic Nanoclusters, ACS Sensors (2022). DOI: 10.1021/acssensors.2c01837
Fei Pan et al, Specific capture of Pseudomonas aeruginosa for rapid detection of antimicrobial resistance in urinary tract infections, Biosensors and Bioelectronics (2022). DOI: 10.1016/j.bios.2022.114962