It’s a major forward-moving step in utilizing extracellular vesicles (EVs) as a demonstrative device. J. Nathaniel Diehl, Ph.D., the lead author of the study, led research at the UNC School of Medicine that demonstrated how a new method for handling extracellular vesicles can significantly enhance the diagnostic and therapeutic potential of disease development.
Cell fragments break off and travel throughout the body as extracellular vesicles (EVs). The significance of their part in the body has come into more clear focus over the most recent couple of years, while already analysts had no clue in the event that these vesicles had any genuine capability or, on the other hand, assuming they were simply “cell flotsam and jetsam.” Now, it is known that EVs deliver proteins, metabolites, and nucleic acids to recipient cells to carry out their biological functions. They also play important roles in the development of diseases like multiple myeloma, pancreatic cancer, and possibly Marfan syndrome, an inherited condition that affects connective tissue.
The workflow for isolation and quantification of plasma EVs—a protocol to assist labs in the field in measuring the quantity and size of EVs in the blood stream of patients—was described in one study that was published in PLOS ONE and was led by first author J. Nathaniel Diehl, Ph.D., a fourth-year medical student at the UNC School of Medicine. The study was titled “A Standardized Method for Plasma Extracellular Vesicle Isolation and Size Distribution Analysis.”
“Our objective is that the procedure will enable our lab or others to build on this research to assist in identifying patients who may need therapy without the use of additional invasive or costly tests.”
J. Nathaniel Diehl, Ph.D., a fourth-year medical student at the UNC School of Medicine,
“Our expectation is that the convention will permit our lab or others to expand on this work to assist with recognizing patients who might require treatment without other obtrusive or costly tests,” Diehl said. “Rather, they could have a little sample of their blood drawn, and the lab could test their blood for EVs. The hospital or clinic does not yet have access to this technology. Nevertheless, this protocol serves as a foundation for that technology,” he stated.
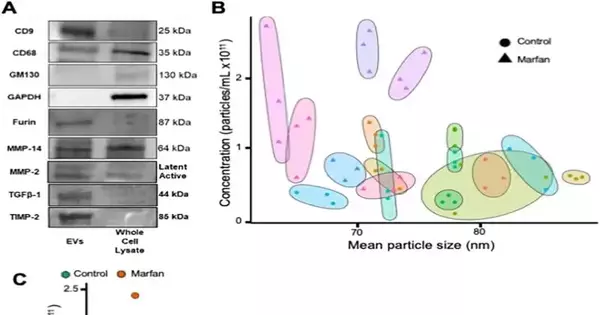
As an example of the indicative potential, Diehl and his group of specialists zeroed in on patients with Marfan disorder with aortic aneurysms and showed that these patients have flowing EVs that are more modest and more bountiful than sound patients.
Technical replicates (two individuals running the same sample) and different personnel (one person running the same sample multiple times) also had highly consistent results. According to Diehl, these results significantly increased their confidence in the method and potential applications of this new protocol.
According to Diehl, “This work is a big step forward for using EVs as a possible diagnostic tool in the clinic.” A method that can be used to answer any number of clinical questions about circulating EVs has been defined and simplified by our team.”
As an individual under Adam W. Akerman, Ph.D., and furthermore, John S. Ikonomidis, MD, Ph.D., at the UNC Institute of Medication, Diehl and his’ group will probably grasp the basic science and pathology that prompt the movement of aortic aneurysms, with an emphasis on patients with connective tissue issues like Marfan’s condition. They hope to use this knowledge to define ways to diagnose aortic aneurysms earlier and potential treatments that could treat them or stop them from getting worse.
According to Diehl, “This work is a launching point for studying EVs and their role in this disease process.” It’s an important first step toward making a blood-based test for diagnosing and monitoring aortic aneurysms. We could potentially see applications of this method as a means of monitoring patients with known aneurysms in order to avoid costly imaging, despite the fact that this field is still new and requires additional research.
According to Diehl, existing imaging methods, such as computed tomography (CT) scans, provide a lot of information about blood vessels and anatomical structures that is necessary for diagnosing aneurysms. Notwithstanding, they likewise have disadvantages, including radiation openness, especially when a patient requires routine follow-up of the aneurysm. With this original convention, specialists are presently ready to frame a strategy, beginning with a blood draw and continuing through the examination of the information. Diehl said that at present, accessible writing has no conventions or procedures that illustrate accepted procedures to this degree.
“It is not yet clear whether EVs contribute straightforwardly to the movement of aortic aneurysms,” said Diehl. “Our research essentially demonstrates that patients with Marfan syndrome who also have aortic aneurysms have EVs that circulate in the blood stream at a higher concentration and are smaller. Future work from our lab and others will ideally characterize the particular jobs of flagging proteins and nucleic acids held inside EVs.”
More information: J. Nathaniel Diehl et al, A standardized method for plasma extracellular vesicle isolation and size distribution analysis, PLOS ONE (2023). DOI: 10.1371/journal.pone.0284875