The event of synthetic responses between like-charged intensities in watery arrangements is extremely delayed since particles repel one another. A new advancement in Nature Correspondences shows a better approach to controlling synthetic responses through charge balance and the expansion of powerful centralizations of reactants. The joint examination groups, led by Prof. Robert Hoyst from the Establishment of Actual Science and the Clean Foundation of Sciences, found that utilizing compounds with an enormous surface energy thickness sped the responses up to a 5-million overlay.
The amalgamation of new materials, or the fabrication of materials, is extremely mind-boggling on the sub-atomic level. We should consider two atoms that have like charges. Because of the shock, they stay a long way from one another and rarely come close enough for the response to occur. This sort of response in unadulterated water can take days or even a long time to finish, but its rate can be radically abbreviated with a touch of flash. Such a flash comes from normal impetuses, similar to compounds or manufactured nanozymes that copy regular ones, as well as metal-based impetuses generally utilized in industry. Regardless of their starting points, they help the flow of responses. In any case, they are typically particular about explicit responses.
As of late, a joint examination group from the Foundation for Actual Science, the Clean Institute of Sciences, the College of Zurich, Radboud College, Lancaster College, the College of Oxford, and the College of Warsaw led by prof. Robert Hoyst exhibited that the speed increase of the response rates between two like-charged organically dynamic particles relies upon the nature and type of the charge in the fluid arrangement.
“The experimental data aided in the development of a predictive theoretical model. The rise in the rate of substrate encounters is the catalytic mechanism. We are convinced that it applies to other particles as well.”
Dr. Paweł Zuk
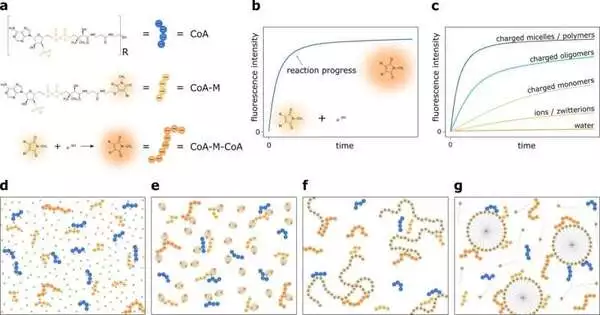
The complicated development of two adversely charged compounds—the coenzyme A particle and its subordinate spurning each other—was investigated as the model response.When compared to natural solvents, this cycle is much slower in fluid media.As a result, it was presented to counter-charged builds in order to support the response rate in water.
The researchers checked particles of various sizes and charge amounts, like particles, monomers, zwitterions, oligomers, polymers, and even micelles. The outcomes were noteworthy. The response rate was expanded 5 million times by the positive-charge micelles. Conversely, monomers, oligomers, and polymers improved the response rate by 103–105 overlays, while particles or zwitterions expanded the beat of the response by just 1000 folds (i.e., traditional screening).
Adam Kowalski comments, “In this work, we expected to answer whether the response rate upgrade by “counter-charged” species could be an overall peculiarity and make sense of the hypothetical premise of such electrostatic catalysis.” “Our paper shows that response rates can be controlled to within a few significant degrees by tuning the extent and spatial conveyance of the electric charge of the impetus.”
How can it function? The substitution of Coenzyme A (CoA) or methylmaleimide for Coenzyme A (CoA-M) in inversely charged mixtures upsets the repugnance between negatively charged CoA and CoA-M.When the size of added compounds is comparable to or greater than the Debye length, negatively charged particles of explicit fixations disregard the shock in decidedly charged specialists, drawing closer to one another.
Thus, the additional compounds screen the particles’ repellent powers, making this interaction a particular instance of catalysis. Experts also tried particle-delicate DNA hybridization to see if the described effect works for more complex cycles.Comparably to the CoA and CoA-M responses, a decidedly energized state speeds up the response rate, affirming the electrostatic idea of catalysis.
“We speed up the synthetic response in water by 5 million times utilizing a strange but basic methodology: emphatically charged surfactants (so essentially a cleanser).” This peculiarity was demonstrated by a covalent response between two coenzymes and the formation of a non-covalent complex—the DNA duplex.We are enabling physicists and organic chemists to accelerate other observed delayed responses between like-charged intensities.Furthermore, our discoveries can open the pathway for completing specific responses in water with surfactants that, at this point, were acting in natural solvents,” claims Dr. Grzegorz Bubak.
As the micelles of cetrimonium chloride (CTAC), benzethonium chloride (BTC), and cetylpyridinium chloride (CPC) are a lot bigger than those of the other multi-charged mixtures and surfactants, their surface charge makes them “contact points” for different particles. This impact is seen in compound responses and dimerization of DNA, including the collaboration of two adversely charged atoms.
Along these lines, this interesting synergist impact can likewise be accomplished for actual cycles upgrading particles’ vehicles. The trial results introduced by specialists demonstrate that the ascent of the response rate is directly associated with the impetus’ surface charge in the framework. Consequently, controlling the compound’s charges empowers controlling the energy of explicit cycles.
Dr. According to Pawe Zuk, “The trial results directed a prescient hypothetical model.” The reactant system is the acceleration of substrate experiences.”We are certain that it is pertinent to different particles as well.”
This advanced technique brings us closer to future detecting applications that will allow us to identify explicit particles in exceptionally weak arrangements or collaborations between specific atoms. Specialists likewise feature the significance of interdisciplinary coordination. Their joint discoveries pave the way for controlling synthetic responses, even within a small scope.
More information: Adam Kowalski et al, Effective screening of Coulomb repulsions in water accelerates reactions of like-charged compounds by orders of magnitude, Nature Communications (2022). DOI: 10.1038/s41467-022-34182-z