Microplastics—little plastic pieces under five millimeters long—are turning into a pervasive natural toxin. Studies propose that all alone, these smidgens are possibly unsafe, and it is unclear what impact they could have on toxins that lock onto them. Presently, analysts detailing their findings in Natural Science and Innovation Letters show that, when combined with microplastics, UV channels utilized in items, for example, sunscreens, can make chromium metal more harmful.
Since microplastics can gather other natural toxins on their surfaces, for example, heavy metals or natural particles, they could present a much bigger issue for untamed life, plants, or people than initially suspected. Past examination has demonstrated the way that weighty metals can undoubtedly join with microplastics and that this mix might actually hurt ocean life.
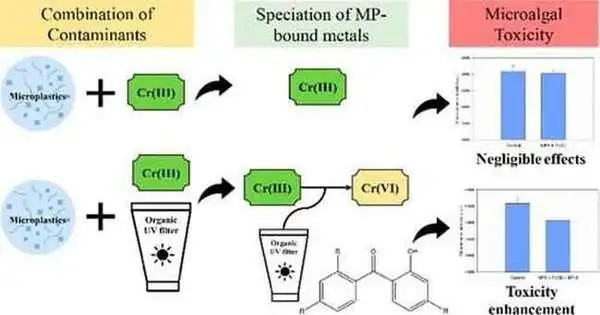
However, in addition to adhering to various impurities, microplastics and the mixed drink of substances on them may interact with one another, changing their compound properties.For instance, certain metals, like chromium (Cr), could take on various oxidation states while on the surfaces of microplastics. Furthermore, while Cr(III) is somewhat protected, Cr(VI) is hazardous.
Thus, Kelvin Sze-Yin Leung and colleagues needed to investigate how the oxidation province of Cr could change when bound to microplastics, as well as what this could mean for a common natural impurity: UV channel particles.
The analysts made combinations of Cr and polystyrene microplastic particles both with and without benzophenone-type UV channels. The researchers discovered that microplastics could contain significantly more Cr when exposed to an UV channel. Also, the oxidation province of Cr was higher in the blends containing the channels.
At last, the group tried to determine whether this expanded oxidation state meant natural harmfulness for a population of microalgae. The microalgae’s development was hindered when presented with the blend containing the channel atom, suggesting that Cr was currently in its more harmful structure. As per the scientists, this implies that microplastics can support changing toxins into a more unsafe structure—a formerly dubious connection.
More information: Wai-Kit Ho et al, Sorption Behavior, Speciation, and Toxicity of Microplastic-Bound Chromium in Multisolute Systems, Environmental Science & Technology Letters (2022). DOI: 10.1021/acs.estlett.2c00689
Journal information: Environmental Science & Technology Letters