Biofilms form when, for example, specific kinds of microbes stick to the outer layer of items in a wet climate and start to repeat, bringing about the discharge of a foul, paste-like substance.
These biofilms aren’t simply unsavory and unappealing anyway; they can be truly irksome. For instance, in the clinical field, the development of biofilm can lessen the adequacy of anti-toxin medicines. The key to understanding biomass development lies in understanding how microbes act as a group.
Another paper published in The European Actual Diary E by Heinrich-Heine-Universität, Düsseldorf, Germany, by scientist Davide Breoni and his co-creators presents a numerical model for the movement of microbes that incorporates cell division and demise, the basic elements of the cell cycle.
“Our novel model belongs to a class of ‘active matter’ theories that are now generating a lot of interest in statistical physics. This field investigates the collective properties of particle systems with their own energy source, such as bacteria.”
Researcher Davide Breoni
The group fostered a numerical model of bacterial development, making a connection between factual material science and biophysics all the while.
“Our new model has a place with a class of models for “dynamic matter” that, as of now, experience a ton of interest in factual material science,” Breoni says. “This field concentrates on the aggregate properties of molecule frameworks that have their own energy source—microbes are a model case.”
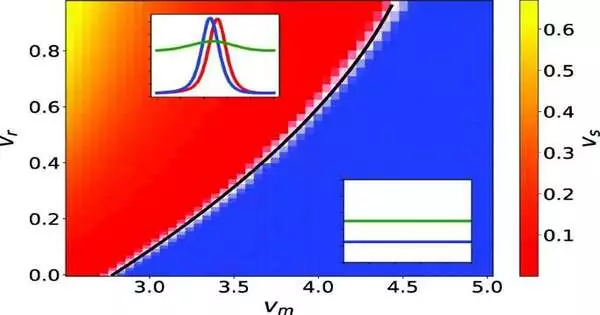
The model conceived by the group conveyed a shock by proposing that, with regards to development, microbes can go about as a unit.
“Over our examination, we figured out that the model predicts that the arrangement of bacterial states can happen through the development of voyaging waves, concentrated “bundles” of microbes,” Breoni adds. “We didn’t anticipate that this should emerge from such a basic model as our own.”
He accepts that the outcomes ought to be fascinating to the overall population, which might know about bacterial states but not how they move in an aggregate manner.
Breoni concludes by stating that this is a basic model for how the exploration could proceed from here. “We could attempt to make the model more sensible and put the outcomes to the test to test its forecasts,” he says. “On the other hand, this exploration is a lot of curiosity driven and results from extreme conversations among the scientists—a methodology we might want to keep up with so we can keep surprising ourselves with our discoveries.”
More information: Davide Breoni et al, A one-dimensional three-state run-and-tumble model with a ‘cell cycle’, The European Physical Journal E (2022). DOI: 10.1140/epje/s10189-022-00238-7
Journal information: European Physical Journal E