The TGF-ß cell flagging organization, vital for different capabilities in all metazoans and engaged with numerous serious human pathologies like immune system illnesses and diseases, is surprisingly adaptable.
Scientists at the MPI for Neurobiology of Conduct and the MPI for Science found an obscure hereditary fluctuation in this flagging pathway among various nematode species, bringing about morphological and social varieties. This new view on the TGF-ß hardware, distributed in Atomic Science and Development, is significant for grasping the advancement of flagging pathways, their versatility to get novel capabilities, and for new systems to control parasitic nematodes.
Have you at any point pondered what you share, practically speaking, with small roundworms? As the “nematodes” are metazoan creatures, as are we, it is a ton. We have comparable organ frameworks, and what we gain from nematodes about quality capability might be directly applicable to human turns of events and illness. The best-perceived species is Caenorhabditis elegans, and one of the many inquiries researchers explore in this model species is “cell flagging,” or how particles cooperate to control a cell’s capability.
A vital messenger protein or “cytokine” is the multifunctional Changing Development Factor-ß (TGF-ß), which is emitted by numerous cell types in both vertebrates and spineless creatures. Through its intricate flagging organization, it manages quality articulation and assumes a vital role all through the lifetime of a creature for improvement, maturing, digestion, and resistance. It isn’t to be expected that a glitch in these pathways can prompt extreme pathologies like rheumatic or cardiovascular illnesses or diseases, and thusly, meddling specialists, such as TGF-ß inhibitors, are now utilized as therapeutics.
“Our results demonstrate a remarkable and hitherto unrecognized flexibility in the TGF-ß signaling pathway between worms. In order to truly understand signaling networks, how they influence behavioral differences between species, and how complex features emerge, we need to step beyond the box and investigate signaling networks in other less conventional model species.”
Dr. James Lightfoot who leads the research group “Genetics of Behavior” at the MPINB.
TGF-ß has recently been discovered to play an important role in some serious Coronavirus diseases, where an ongoing safe response fosters that is not generally coordinated against the actual infection, but rather against the patient’s own body.
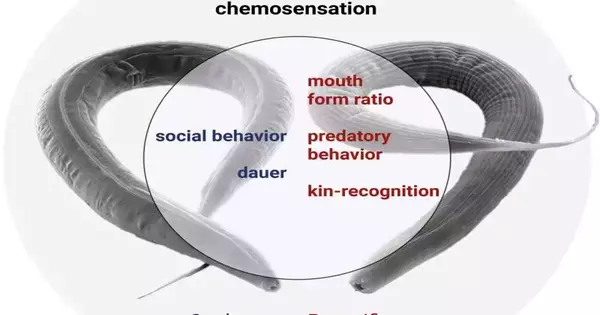
Striking fluctuations in conduct and morphology
Analysts at the Maximum Planck Foundation for Neurobiology of Conduct (MPINB) in Bonn, the Maximum Planck Organization for Science in Tübingen, and the California State College have now acquired new insights into the development and capability of TGF-ß flagging. They recognized and analyzed the TGF-ß qualities across nine different nematode species, which uncovered amazing contrasts in the quantity of TGF-ß qualities across various species.
The group then focused on the species Pristionchus pacificus, which showed numerous variations in the amount of TGF-ß qualities when compared to other nematodes, including C. elegans.By making transformations in various pieces of the flagging pathway utilizing hereditary devices like CRISPR/Cas9, they observed that there were numerous startling differences in capability between P. pacificus and C. elegans.
They showed that the supposed DBL-1 pathway for managing body morphology shows up profoundly saved, while they tracked down a striking fluctuation in the capability of the purported DAF-7 pathway. This revealed significant differences for improvement in natural detection and behavior between these species.
The scientists tracked down that TGF-ß flagging vitally affects significant aggregates in P. pacificus. Whereas C. elegans only feeds on microbes, P. pacificus is an omnivore that can hatch from other nematode hatchlings.Moreover, they have a family acknowledgment framework that shields their posterity from being eaten. This study found that TGF-ß motion in P. pacificus is important for framing the mouth structures associated with predation as well as for laying out the family sign to recognize and protect their family members.
“Our discoveries show a formerly obscure and amazing adaptability in the TGF-ß flagging pathway across nematodes.” “We want to consider and investigate flagging organizations in other less common model species to truly comprehend their capability, how they direct social contrasts between the species, and how complex qualities develop,” says Dr. James Lightfoot, who leads the examination bunch “Hereditary Qualities of Conduct” at the MPINB.
New experiences could assist with controlling unsafe parasites.
Helminthiasis, a parasitic worm disease, is a major concern for human and animal health, and protection from existing antihelmintics is required.Significantly, free-living nematodes such as C. elegans and P. pacificus can provide valuable experiences for furthering our understanding of parasitic nematodes because they share many similarities with their parasitic cousins.
Specifically, these free-living species can enter an enduring and stress-safe formative stage called the “dauer” structure when they experience unfriendly circumstances, and this has numerous likenesses with the infective hatchlings of parasitic nematodes. Thusly, a ton of examination has been centered around the dauer stage in C. elegans, and TGF-ß flagging has been viewed as significant for dauer arrangement.
“In P. pacificus, we didn’t track down a similar impact.” “Our outcomes propose that the system found in C. elegans could not really be similar in different nematodes,” says Dr. James Lightfoot. Understanding the previously obscure inconsistency between various species is thus critical for the development of new helpful methodologies against dangerous parasites.
More information: Wen-Sui Lo et al, Evolution and diversity of TGF-ß pathways are linked with novel developmental and behavioural traits, Molecular Biology and Evolution (2022). DOI: 10.1093/molbev/msac252
Journal information: Molecular Biology and Evolution